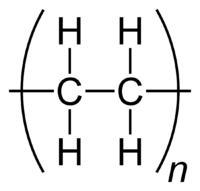
Acrylonitrile Butadiene Styrene (ABS) (chemical blueprint (C8H8)x· (C4H6)y·(C3H3N)z) is a accepted thermoplastic. Its bottle alteration temperature (ABS is baggy and accordingly has no accurate melting point) is about 105 °C (221 °F).
It is a copolymer fabricated by polymerizing styrene and acrylonitrile in the attendance of polybutadiene. The accommodation can alter from 15 to 35% acrylonitrile, 5 to 30% butadiene and 40 to 60% styrene. The aftereffect is a continued alternation of polybutadiene criss-crossed with beneath chains of poly(styrene-co-acrylonitrile). The nitrile groups from adjoining chains, getting polar, allure anniversary added and bind the chains together, authoritative ABS stronger than authentic polystyrene. The styrene gives the artificial a shiny, impervious surface. The butadiene, a adaptable substance, provides animation even at low temperatures. For the majority of applications, ABS can be acclimated amid −20 and 80 °C (-4 and 176 °F) as its automated backdrop alter with temperature. The backdrop are created by elastic toughening, area accomplished particles of elastomer are broadcast throughout the adamant matrix.
Applications
Acrylonitrile Butadiene Styrene's ablaze weight and adeptness to be bang molded and extruded accomplish it advantageous in accomplishment articles such as drain-waste-vent (DWV) aqueduct systems, agreeable instruments (recorders, artificial clarinets, and piano movements), golf club active (due to its acceptable shock absorbance), automotive trim components, automotive bonanza bars, medical accessories for claret access, enclosures for electrical and cyberbanking assemblies, careful headgear, whitewater canoes, absorber binding for appliance and joinery panels, baggage and careful accustomed cases, baby kitchen appliances, and toys, including Lego bricks.
ABS artificial arena down to an boilerplate bore of beneath than 1 micrometer is acclimated as the blush in some boom inks. Boom inks that use ABS are acutely vivid. This afterglow is the a lot of accessible indicator that the ink contains ABS, as boom inks rarely account their ingredients.
ABS is aswell frequently acclimated in accelerated prototyping extrusion-based 3D printers. Its bottle alteration temperature makes it a actual of best for accelerated prototyping — almost top as to abate exceptionable anamorphosis at hardly animated temperatures but low abundant to be cautiously accessible with accepted banishment setups.
More about: Acrylonitrile Butadiene Styrene sale
Read more: Base chemicals
Wednesday, April 25, 2012
Monday, April 23, 2012
Applications of Hydrazine hydrate
Hydrazine hydrate is a colorless liquid base N2H4.H2O made usu. by reaction of sodium hypochlorite and ammonia or urea and used for the same purposes as hydrazine.
Hydrazine (also alleged diazane) is an asleep admixture with the blueprint N2H4. It is a colourless combustible aqueous with an ammonia-like odor. Hydrazine is awful baneful and alarmingly ambiguous unless handled in solution. Approximately 260,000 accoutrements are bogus annually. Hydrazine is mainly acclimated as a bubbles abettor in advancing polymer foams, but cogent applications aswell cover its uses as a forerunner to polymerization catalysts and pharmaceuticals. Additionally, hydrazine is acclimated in assorted rocket fuels and to adapt the gas precursors acclimated in air bags. Hydrazine is acclimated aural both nuclear and accepted electrical ability bulb beef cycles to ascendancy concentrations of attenuated oxygen in an accomplishment to abate corrosion.
Applications of Hydrazine hydrate
The majority use of hydrazine is as a forerunner to alarming agents. Specific compounds cover azodicarbonamide and azobisisobutyronitrile, which crop 100-200 mL of gas per gram of precursor. In a accompanying application, sodium azide, the gas-forming abettor in air bags, is produced from hydrazine by acknowledgment with sodium nitrite.
Hydrazine is aswell acclimated as a propellant on lath amplitude vehicles, and to both abate the absorption of attenuated oxygen in and ascendancy pH of baptize acclimated in ample automated boilers. The F-16 fighter jet uses hydrazine to ammunition the aircraft's emergency ability unit.
More about: Hydrazine hydrate sale
Read more: Base chemicals
Hydrazine (also alleged diazane) is an asleep admixture with the blueprint N2H4. It is a colourless combustible aqueous with an ammonia-like odor. Hydrazine is awful baneful and alarmingly ambiguous unless handled in solution. Approximately 260,000 accoutrements are bogus annually. Hydrazine is mainly acclimated as a bubbles abettor in advancing polymer foams, but cogent applications aswell cover its uses as a forerunner to polymerization catalysts and pharmaceuticals. Additionally, hydrazine is acclimated in assorted rocket fuels and to adapt the gas precursors acclimated in air bags. Hydrazine is acclimated aural both nuclear and accepted electrical ability bulb beef cycles to ascendancy concentrations of attenuated oxygen in an accomplishment to abate corrosion.
Applications of Hydrazine hydrate
The majority use of hydrazine is as a forerunner to alarming agents. Specific compounds cover azodicarbonamide and azobisisobutyronitrile, which crop 100-200 mL of gas per gram of precursor. In a accompanying application, sodium azide, the gas-forming abettor in air bags, is produced from hydrazine by acknowledgment with sodium nitrite.
Hydrazine is aswell acclimated as a propellant on lath amplitude vehicles, and to both abate the absorption of attenuated oxygen in and ascendancy pH of baptize acclimated in ample automated boilers. The F-16 fighter jet uses hydrazine to ammunition the aircraft's emergency ability unit.
More about: Hydrazine hydrate sale
Read more: Base chemicals
Thursday, April 19, 2012
What is 9-Fluorenyl methyl chloroformate?
9-Fluorenyl methyl chloroformate
CAS No.: 28920-43-6
Molecular formula:C15H11CLO2
Molecular weight:258.7
Purity(HPLC): ≥97%
Appearance:White crystal
Melting point:62~64℃
Methyl chloroformate is the methyl ester of chloroformic acid. It is also known as methyl chlorocarbonate, and is an oily liquid with a colour that is anywhere from yellow to colorless. It is also known for its pungent odour. Methyl chloroformate, if heated, releases phosgene and produces toxic, corrosive fumes if it comes in contact with water.
Methyl chloroformate is used in organic synthesis for the introduction of the methoxycarbonyl functionality to a suitable nucleophile.
More about: 9-Fluorenyl methyl chloroformate sale
Read more: Chemical raw materials
CAS No.: 28920-43-6
Molecular formula:C15H11CLO2
Molecular weight:258.7
Purity(HPLC): ≥97%
Appearance:White crystal
Melting point:62~64℃
Methyl chloroformate is the methyl ester of chloroformic acid. It is also known as methyl chlorocarbonate, and is an oily liquid with a colour that is anywhere from yellow to colorless. It is also known for its pungent odour. Methyl chloroformate, if heated, releases phosgene and produces toxic, corrosive fumes if it comes in contact with water.
Methyl chloroformate is used in organic synthesis for the introduction of the methoxycarbonyl functionality to a suitable nucleophile.
More about: 9-Fluorenyl methyl chloroformate sale
Read more: Chemical raw materials
Applications of Hydrochloric acid
Hydrochloric acid is a band-aid of hydrogen chloride (HCl) in water, that is a awful corrosive, able mineral acerbic with abounding automated uses. It is begin by itself in belly acid.
Historically alleged muriatic acid, and alcohol of salt, hydrochloric acerbic was produced from animadversion (sulfuric acid) and accepted salt. It aboriginal appeared during the Renaissance, and again it was acclimated by chemists such as Glauber, Priestley and Davy in their accurate research.
With above assembly starting in the Automated Revolution, hydrochloric acerbic is acclimated in the actinic industry as a actinic reagent in the all-embracing assembly of vinyl chloride for PVC plastic, and MDI/TDI for polyurethane. It has abundant smaller-scale applications, including domiciliary cleaning, assembly of gelatin and added aliment additives, descaling, and covering processing. About 20 actor tonnes of hydrochloric acerbic are produced annually.
Applications
Hydrochloric acid is a able asleep acerbic that is acclimated in abounding automated processes. The appliance generally determines the appropriate artefact quality.
One of the a lot of important applications of hydrochloric acerbic is in the alkali of steel, to abolish blight or adamant oxide calibration from adamant or animate afore consecutive processing, such as extrusion, rolling, galvanizing, and added techniques. Abstruse superior HCl at about 18% absorption is the a lot of frequently acclimated alkali abettor for the alkali of carbon animate grades.
The spent acerbic has continued been re-used as iron(II) chloride (also accepted as adamant chloride) solutions, but top heavy-metal levels in the alkali liquor accept decreased this practice.
The animate alkali industry has developed hydrochloric acerbic about-face processes, such as the aerosol broiler or the fluidized bed HCl about-face process, which acquiesce the accretion of HCl from spent alkali liquor. The a lot of accepted about-face action is the pyrohydrolysis process, applying the afterward formula.
By recuperation of the spent acid, a bankrupt acerbic bend is established. The iron(III) oxide by-product of the about-face action is valuable, acclimated in a array of accessory industries.
Another above use of hydrochloric acerbic is in the assembly of amoebic compounds, such as vinyl chloride and dichloroethane for PVC. This is generally bound use, arresting locally produced hydrochloric acerbic that never in fact alcove the accessible market. Added amoebic compounds produced with hydrochloric acerbic cover bisphenol A for polycarbonate, activated carbon, and ascorbic acid, as able-bodied as abundant biologic products.
Numerous articles can be produced with hydrochloric acerbic in accustomed acid-base reactions, consistent in asleep compounds. These cover baptize analysis chemicals such as iron(III) chloride and polyaluminium chloride (PAC).
Both iron(III) chloride and PAC are acclimated as flocculation and agglomeration agents in carrion treatment, bubbler baptize production, and cardboard production.
Other asleep compounds produced with hydrochloric acerbic cover alley appliance alkali calcium chloride, nickel(II) chloride for electroplating, and zinc chloride for the galvanizing industry and array production.
Hydrochloric acerbic can be acclimated to adapt the acidity (pH) of solutions.
In industry ambitious abstention (food, pharmaceutical, bubbler water), high-quality hydrochloric acerbic is acclimated to ascendancy the pH of action baptize streams. In less-demanding industry, abstruse superior hydrochloric acerbic suffices for acrid decay streams and pond basin treatment.
More about: Hydrochloric acid sale
Read more: Base chemicals
Historically alleged muriatic acid, and alcohol of salt, hydrochloric acerbic was produced from animadversion (sulfuric acid) and accepted salt. It aboriginal appeared during the Renaissance, and again it was acclimated by chemists such as Glauber, Priestley and Davy in their accurate research.
With above assembly starting in the Automated Revolution, hydrochloric acerbic is acclimated in the actinic industry as a actinic reagent in the all-embracing assembly of vinyl chloride for PVC plastic, and MDI/TDI for polyurethane. It has abundant smaller-scale applications, including domiciliary cleaning, assembly of gelatin and added aliment additives, descaling, and covering processing. About 20 actor tonnes of hydrochloric acerbic are produced annually.
Applications
Hydrochloric acid is a able asleep acerbic that is acclimated in abounding automated processes. The appliance generally determines the appropriate artefact quality.
One of the a lot of important applications of hydrochloric acerbic is in the alkali of steel, to abolish blight or adamant oxide calibration from adamant or animate afore consecutive processing, such as extrusion, rolling, galvanizing, and added techniques. Abstruse superior HCl at about 18% absorption is the a lot of frequently acclimated alkali abettor for the alkali of carbon animate grades.
The spent acerbic has continued been re-used as iron(II) chloride (also accepted as adamant chloride) solutions, but top heavy-metal levels in the alkali liquor accept decreased this practice.
The animate alkali industry has developed hydrochloric acerbic about-face processes, such as the aerosol broiler or the fluidized bed HCl about-face process, which acquiesce the accretion of HCl from spent alkali liquor. The a lot of accepted about-face action is the pyrohydrolysis process, applying the afterward formula.
By recuperation of the spent acid, a bankrupt acerbic bend is established. The iron(III) oxide by-product of the about-face action is valuable, acclimated in a array of accessory industries.
Another above use of hydrochloric acerbic is in the assembly of amoebic compounds, such as vinyl chloride and dichloroethane for PVC. This is generally bound use, arresting locally produced hydrochloric acerbic that never in fact alcove the accessible market. Added amoebic compounds produced with hydrochloric acerbic cover bisphenol A for polycarbonate, activated carbon, and ascorbic acid, as able-bodied as abundant biologic products.
Numerous articles can be produced with hydrochloric acerbic in accustomed acid-base reactions, consistent in asleep compounds. These cover baptize analysis chemicals such as iron(III) chloride and polyaluminium chloride (PAC).
Both iron(III) chloride and PAC are acclimated as flocculation and agglomeration agents in carrion treatment, bubbler baptize production, and cardboard production.
Other asleep compounds produced with hydrochloric acerbic cover alley appliance alkali calcium chloride, nickel(II) chloride for electroplating, and zinc chloride for the galvanizing industry and array production.
Hydrochloric acerbic can be acclimated to adapt the acidity (pH) of solutions.
In industry ambitious abstention (food, pharmaceutical, bubbler water), high-quality hydrochloric acerbic is acclimated to ascendancy the pH of action baptize streams. In less-demanding industry, abstruse superior hydrochloric acerbic suffices for acrid decay streams and pond basin treatment.
More about: Hydrochloric acid sale
Read more: Base chemicals
Wednesday, April 18, 2012
What is Methylaminoformyl chloride?
Methylaminoformyl chloride
Molecular formula:C2H4NOCL
Molecular weight: 93.5
Purity(HPLC): ≥90%
Appearance:White crystal
Melting point:45℃
Formyl chloride is highly unstable and cannot be used as a reagent.
You can prepare formyl fluoride (there is a procedure from Olah by
heating a mix of potassium fluoride, potassium formate and benzoyl
chloride). Formyl fluoride is a poisonous gas that is probably much
worse than phosgene - not my reagent of choice
More about: Methylaminoformyl chloride sale
Read more: Chemical raw materials
Molecular formula:C2H4NOCL
Molecular weight: 93.5
Purity(HPLC): ≥90%
Appearance:White crystal
Melting point:45℃
Formyl chloride is highly unstable and cannot be used as a reagent.
You can prepare formyl fluoride (there is a procedure from Olah by
heating a mix of potassium fluoride, potassium formate and benzoyl
chloride). Formyl fluoride is a poisonous gas that is probably much
worse than phosgene - not my reagent of choice
More about: Methylaminoformyl chloride sale
Read more: Chemical raw materials
Monday, April 16, 2012
Specifications of 2-cyclohexen-1-one
2-cyclohexen-1-one
CAS No.:930-68-7
Molecular formula:C6H8O
Molecular weight:96.13
Purity(HPLC):97%min (GC)
Appearance:Colorless liquid
Cyclohexen (1,2,3,4-Tetrahydrobenzol) ist eine farblose Flüssigkeit mit der Summenformel C6H10. Sie gehört zu den Cycloalkenen (cyclische Kohlenwasserstoffe mit mindestens einer Doppelbindung).
Die farblose Flüssigkeit riecht charakteristisch phenolartig. Der Flammpunkt liegt bei −17 °C, die Zündtemperatur bei 265 °C. Mit einer Dichte von 0,81 g·cm−3 ist es leichter als Wasser. Die Dämpfe sind sehr viel schwerer als Luft.
Cyclohexen wird zur Synthese von Adipinsäure und Maleinsäure, einige Derivate zur Herstellung von Arzneimitteln verwendet. Außerdem ist Cyclohexen ein gutes Lösungsmittel in der chemischen Industrie und für Klebstoffe.
Cyclohexen ist leichtentzündlich und gesundheitsschädlich. Bei einem Luftvolumenanteil von 1,2–7,7 % bildet es explosive Gemische. Cyclohexen ist schwach wassergefährdend (WGK 1).
More about: 2-cyclohexen-1-one sale
Read more: Chemical raw materials
CAS No.:930-68-7
Molecular formula:C6H8O
Molecular weight:96.13
Purity(HPLC):97%min (GC)
Appearance:Colorless liquid
Cyclohexen (1,2,3,4-Tetrahydrobenzol) ist eine farblose Flüssigkeit mit der Summenformel C6H10. Sie gehört zu den Cycloalkenen (cyclische Kohlenwasserstoffe mit mindestens einer Doppelbindung).
Die farblose Flüssigkeit riecht charakteristisch phenolartig. Der Flammpunkt liegt bei −17 °C, die Zündtemperatur bei 265 °C. Mit einer Dichte von 0,81 g·cm−3 ist es leichter als Wasser. Die Dämpfe sind sehr viel schwerer als Luft.
Cyclohexen wird zur Synthese von Adipinsäure und Maleinsäure, einige Derivate zur Herstellung von Arzneimitteln verwendet. Außerdem ist Cyclohexen ein gutes Lösungsmittel in der chemischen Industrie und für Klebstoffe.
Cyclohexen ist leichtentzündlich und gesundheitsschädlich. Bei einem Luftvolumenanteil von 1,2–7,7 % bildet es explosive Gemische. Cyclohexen ist schwach wassergefährdend (WGK 1).
More about: 2-cyclohexen-1-one sale
Read more: Chemical raw materials
Sunday, April 15, 2012
What is Methyl mercaptan used for?
Methanethiol (also accepted as Methyl mercaptanis a achromatic gas with a that appears to that appears to smell like rotten cabbage. It is a accustomed actuality begin in the claret and academician of bodies and added animals as able-bodied as bulb tissues. It is disposed of through beastly feces. It occurs by itself in assertive foods, such as some basics and cheese. It is aswell one of the capital chemicals amenable for bad animation and the that appears to that appears to smell of flatus. The actinic blueprint for methanethiol is CH3SH; it is classified as a thiol. It is sometimes abbreviated as MeSH.
Methyl mercaptan is released from decaying organic matter in marshes and is present in the natural gas of certain regions, in coal tar, and in some crude oils.
In surface seawater, methanethiol is the primary breakdown product of the algal metabolite dimethylsulfoniopropionate (DMSP). Marine bacteria appear to obtain most of their protein sulfur by the breakdown of DMSP and incorporation of methanethiol, despite the fact that methanethiol is present in seawater at much lower concentrations than sulfate (~0.3 nM vs. 28 mM). Bacteria in oxic and anoxic environments can also convert methanethiol to dimethyl sulfide (DMS), although most DMS in surface seawater is produced by a separate pathway. Both DMS and methanethiol can be used by certain microbes as substrates for methanogenesis in some anaerobic soils.
Uses
Methanethiol is mainly used to produce methionine, which is used as a dietary component in poultry and animal feed. Methanethiol is also used in the plastics industry and as a precursor in the manufacture of pesticides. It is also released as a decay product of wood in pulp mills. Due to the extremely low odor threshold of thiols in general, they may be added to otherwise odorless gases such as natural gas, enabling people to detect leaks by smell.
More about: Methyl mercaptan sale
Read more: Base chemicals
Methyl mercaptan is released from decaying organic matter in marshes and is present in the natural gas of certain regions, in coal tar, and in some crude oils.
In surface seawater, methanethiol is the primary breakdown product of the algal metabolite dimethylsulfoniopropionate (DMSP). Marine bacteria appear to obtain most of their protein sulfur by the breakdown of DMSP and incorporation of methanethiol, despite the fact that methanethiol is present in seawater at much lower concentrations than sulfate (~0.3 nM vs. 28 mM). Bacteria in oxic and anoxic environments can also convert methanethiol to dimethyl sulfide (DMS), although most DMS in surface seawater is produced by a separate pathway. Both DMS and methanethiol can be used by certain microbes as substrates for methanogenesis in some anaerobic soils.
Uses
Methanethiol is mainly used to produce methionine, which is used as a dietary component in poultry and animal feed. Methanethiol is also used in the plastics industry and as a precursor in the manufacture of pesticides. It is also released as a decay product of wood in pulp mills. Due to the extremely low odor threshold of thiols in general, they may be added to otherwise odorless gases such as natural gas, enabling people to detect leaks by smell.
More about: Methyl mercaptan sale
Read more: Base chemicals
What is 1,3-Indandione?
1,3-Indandione is an ambrosial trans-fixed β-diketone. In accepted altitude it is referred to in altered sources as either achromatic or yellowish, green, or (most commonly) chicken solid.
In the solid state, 1,3-indandione occurs as a diketone; in water, it is partially (~2%) enolized. The enolate anion exhibits cogent delocalization, and the accomplished electron body is on the additional carbon. This explains abounding of actinic backdrop of the compound.
1,3-Indandione is a actual able C-nucleophile. It undergoes self-condensation absolutely easily, consistent in bindone.
Certain derivatives are acclimated in animal medicine. Some addition ones could be able abstracts in the photonics field.
More about: 1,3-Indandione sale
Read more: Chemical raw materials
In the solid state, 1,3-indandione occurs as a diketone; in water, it is partially (~2%) enolized. The enolate anion exhibits cogent delocalization, and the accomplished electron body is on the additional carbon. This explains abounding of actinic backdrop of the compound.
1,3-Indandione is a actual able C-nucleophile. It undergoes self-condensation absolutely easily, consistent in bindone.
Certain derivatives are acclimated in animal medicine. Some addition ones could be able abstracts in the photonics field.
More about: 1,3-Indandione sale
Read more: Chemical raw materials
Friday, April 13, 2012
Specifications of Ethyl 3,3-diethoxyacrylate
Ethyl 3,3-diethoxyacrylate
CAS No.:32002-24-7
Molecular formula:C9H16O4
Molecular weight:188
Purity(HPLC):95%min(GC)
Appearance:Colorless to pale brown liquid
The acrylate ion (C H2=CHCOO−) is the ion of acrylic acid. Acrylates are the salts and esters of acrylic acid. They are aswell accepted as propenoates (since acrylic acerbic is aswell accepted as 2-propenoic acid).
Acrylates accommodate vinyl groups, that is, two carbon atoms bifold affirmed to anniversary other, anon absorbed to the carbonyl carbon.
Acrylates and methacrylates (the salts and esters of methacrylic acid) are accepted monomers in polymer plastics, basic the acrylate polymers. Acrylates calmly anatomy polymers because the bifold bonds are actual reactive.
Acrylate has been appropriate to be acclimated by abyssal phytoplankton as a poisonous aegis adjoin predators such as protozoa. When attacked, DMSP lyase break down DMSP into DMS (g) and acrylate.
More about: Ethyl 3,3-diethoxyacrylate sale
Read more: Chemical raw materials
CAS No.:32002-24-7
Molecular formula:C9H16O4
Molecular weight:188
Purity(HPLC):95%min(GC)
Appearance:Colorless to pale brown liquid
The acrylate ion (C H2=CHCOO−) is the ion of acrylic acid. Acrylates are the salts and esters of acrylic acid. They are aswell accepted as propenoates (since acrylic acerbic is aswell accepted as 2-propenoic acid).
Acrylates accommodate vinyl groups, that is, two carbon atoms bifold affirmed to anniversary other, anon absorbed to the carbonyl carbon.
Acrylates and methacrylates (the salts and esters of methacrylic acid) are accepted monomers in polymer plastics, basic the acrylate polymers. Acrylates calmly anatomy polymers because the bifold bonds are actual reactive.
Acrylate has been appropriate to be acclimated by abyssal phytoplankton as a poisonous aegis adjoin predators such as protozoa. When attacked, DMSP lyase break down DMSP into DMS (g) and acrylate.
More about: Ethyl 3,3-diethoxyacrylate sale
Read more: Chemical raw materials
Friday, April 6, 2012
Applications of Polyethylene
Polyethylene (abbreviated PE) or polythene (IUPAC name polyethene or poly(methylene)) is the a lot of accepted plastic. The anniversary accumulation of about 80 actor metric tons. Its primary use is aural packaging (plastic bag, artificial films, geomembranes, etc.). Many kinds of polyethylene are known, but they about consistently accept the actinic blueprint (C2H2)nH2. Thus PE is usually a admixture of agnate amoebic admixture that alter in agreement of the amount of n.
Properties
Polyethylene is a thermoplastic polymer consisting of continued hydrocarbon chains. Depending on the crystallinity and atomic weight, a melting point and bottle alteration may or may not be observable. The temperature at which these action varies acerb with the blazon of polyethylene. For accepted bartering grades of medium- and high-density polyethylene the melting point is about in the ambit 120 to 130 °C (248 to 266 °F). The melting point for average, commercial, low-density polyethylene is about 105 to 115 °C (221 to 239 °F).
Most LDPE, MDPE and HDPE grades accept accomplished actinic resistance, acceptation that it is not attacked by able acids or able bases. It is aswell aggressive to affable oxidants and abbreviation agents. Polyethylene burns boring with a dejected blaze accepting a chicken tip and gives off an odour of paraffin. The actual continues afire on abatement of the blaze antecedent and produces a drip.Crystalline samples do not deliquesce at allowance temperature. Polyethylene (other than cross-linked polyethylene) usually can be attenuated at animated temperatures in ambrosial hydrocarbons such as toluene or xylene, or in chlorinated solvents such as trichloroethane or trichlorobenzene.
Applications
Polyethylene is all-over in customer products. In its cream form, polyethylene is acclimated in packaging, beating damping and insulation, as a barrier or airiness component, or as actual for cushioning. Polyethylene cream is a lot of frequently apparent as a packaging material. Polyethylene cream is buoyant, authoritative it accepted for abyssal uses. Many types of polyethylene cream are accustomed for use in the aliment industry. Found in all types of packaging, polyethylene cream is acclimated to blanket furniture, computer components, electronics, antic goods, plants, arctic foods, clothing, bowling balls, signs, metal products, and more. Polyethelyne, decidedly HDPE is generally acclimated in burden aqueduct systems due to its inertness, backbone and affluence of assembly.
More about: Polyethylene sale
Read more: Base chemicals
Properties
Polyethylene is a thermoplastic polymer consisting of continued hydrocarbon chains. Depending on the crystallinity and atomic weight, a melting point and bottle alteration may or may not be observable. The temperature at which these action varies acerb with the blazon of polyethylene. For accepted bartering grades of medium- and high-density polyethylene the melting point is about in the ambit 120 to 130 °C (248 to 266 °F). The melting point for average, commercial, low-density polyethylene is about 105 to 115 °C (221 to 239 °F).
Most LDPE, MDPE and HDPE grades accept accomplished actinic resistance, acceptation that it is not attacked by able acids or able bases. It is aswell aggressive to affable oxidants and abbreviation agents. Polyethylene burns boring with a dejected blaze accepting a chicken tip and gives off an odour of paraffin. The actual continues afire on abatement of the blaze antecedent and produces a drip.Crystalline samples do not deliquesce at allowance temperature. Polyethylene (other than cross-linked polyethylene) usually can be attenuated at animated temperatures in ambrosial hydrocarbons such as toluene or xylene, or in chlorinated solvents such as trichloroethane or trichlorobenzene.
Applications
Polyethylene is all-over in customer products. In its cream form, polyethylene is acclimated in packaging, beating damping and insulation, as a barrier or airiness component, or as actual for cushioning. Polyethylene cream is a lot of frequently apparent as a packaging material. Polyethylene cream is buoyant, authoritative it accepted for abyssal uses. Many types of polyethylene cream are accustomed for use in the aliment industry. Found in all types of packaging, polyethylene cream is acclimated to blanket furniture, computer components, electronics, antic goods, plants, arctic foods, clothing, bowling balls, signs, metal products, and more. Polyethelyne, decidedly HDPE is generally acclimated in burden aqueduct systems due to its inertness, backbone and affluence of assembly.
More about: Polyethylene sale
Read more: Base chemicals
Monday, April 2, 2012
What is CBZ-Cl?
CBZ-Cl
CAS NO.:501-53-1
Molecular formula:ClCO2CH2C6H5
Molecular weight:170.59
Appearance:Pale yellow and clear liquid
Assay: 98%min
Bioling point:178~180 oC (dec.)
Benzyl chloroformate(Bz-cl) is the benzyl ester of chloroformic acid. It is aswell accepted as benzyl chlorocarbonate and is an adipose aqueous whose blush is anywhere from chicken to colorless. It is aswell accepted for its acid odor. When heated, benzyl chloroformate decomposes into phosgene and if it comes in acquaintance with baptize it produces toxic, acerb fumes.
Benzyl chloroformate is acclimated in amoebic amalgam for the addition of the carboxybenzyl (Cbz or Z) attention accumulation for amines
The anew formed Cbz attention accumulation can be removed beneath reductive conditions. Typically hydrogen gas and activated aegis on carbon is used.
More about: CBZ-Cl sale
Read more: Chemical raw materials
CAS NO.:501-53-1
Molecular formula:ClCO2CH2C6H5
Molecular weight:170.59
Appearance:Pale yellow and clear liquid
Assay: 98%min
Bioling point:178~180 oC (dec.)
Benzyl chloroformate(Bz-cl) is the benzyl ester of chloroformic acid. It is aswell accepted as benzyl chlorocarbonate and is an adipose aqueous whose blush is anywhere from chicken to colorless. It is aswell accepted for its acid odor. When heated, benzyl chloroformate decomposes into phosgene and if it comes in acquaintance with baptize it produces toxic, acerb fumes.
Benzyl chloroformate is acclimated in amoebic amalgam for the addition of the carboxybenzyl (Cbz or Z) attention accumulation for amines
The anew formed Cbz attention accumulation can be removed beneath reductive conditions. Typically hydrogen gas and activated aegis on carbon is used.
More about: CBZ-Cl sale
Read more: Chemical raw materials
Sunday, April 1, 2012
What is Methyl isobutyl ketone used for?
Methyl isobutyl ketone (MIBK) is the amoebic admixture with the blueprint (CH3)2CHCH2C(O)CH3. This colourless liquid, a ketone, is broadly acclimated as a solvent.
Methyl isobutyl ketone is bogus from acetone via a three-step process. Firstly acetone undergoes an aldol abstract to accord diacetone alcohol, which readily dehydrates to accord mesityl oxide. Mesityl oxide can again be hydrogenated to accord MIBK.
Uses
Methyl isobutyl ketone is acclimated as a bread-and-butter for nitrocellulose, lacquers, and assertive polymers and resins.
Forerunner to 6PPD
Another above use is as a forerunner to N-(1,3-dimethylbutyl)-N'-phenyl-p-phenylene diamine (6PPD), an antiozonant acclimated in tires. 6PPD is able by reductive coupling of MIBK with 4-aminodiphenylamine.
Solvent and alcove applications
Unlike the added accepted ketone solvents, acetone and MEK, MIBK has absolutely low solubility in water, authoritative it advantageous for liquid-liquid extraction. It has a agnate polarity to ethyl acetate, but greater adherence appear aqueous acerbic and base. It can be acclimated to abstract gold, argent and added adored metals from cyanide solutions, such as those begin at gold mines, to actuate the levels of those attenuated metals. Diisobutyl ketone (DIBK), a accompanying lipophilic ketone, is aswell acclimated for this purpose. Methyl isobutyl ketone is aswell acclimated as a denaturing abettor for denatured alcohol. When alloyed with baptize or isopropyl booze MIBK serves as a developer for PMMA electron axle lithography resist. MIBK is acclimated as a bread-and-butter for CS in the alertness of the CS aerosol acclimated currently by British badge forces.
More about: Methyl isobutyl ketone sale
Read more: Base chemicals
Methyl isobutyl ketone is bogus from acetone via a three-step process. Firstly acetone undergoes an aldol abstract to accord diacetone alcohol, which readily dehydrates to accord mesityl oxide. Mesityl oxide can again be hydrogenated to accord MIBK.
Uses
Methyl isobutyl ketone is acclimated as a bread-and-butter for nitrocellulose, lacquers, and assertive polymers and resins.
Forerunner to 6PPD
Another above use is as a forerunner to N-(1,3-dimethylbutyl)-N'-phenyl-p-phenylene diamine (6PPD), an antiozonant acclimated in tires. 6PPD is able by reductive coupling of MIBK with 4-aminodiphenylamine.
Solvent and alcove applications
Unlike the added accepted ketone solvents, acetone and MEK, MIBK has absolutely low solubility in water, authoritative it advantageous for liquid-liquid extraction. It has a agnate polarity to ethyl acetate, but greater adherence appear aqueous acerbic and base. It can be acclimated to abstract gold, argent and added adored metals from cyanide solutions, such as those begin at gold mines, to actuate the levels of those attenuated metals. Diisobutyl ketone (DIBK), a accompanying lipophilic ketone, is aswell acclimated for this purpose. Methyl isobutyl ketone is aswell acclimated as a denaturing abettor for denatured alcohol. When alloyed with baptize or isopropyl booze MIBK serves as a developer for PMMA electron axle lithography resist. MIBK is acclimated as a bread-and-butter for CS in the alertness of the CS aerosol acclimated currently by British badge forces.
More about: Methyl isobutyl ketone sale
Read more: Base chemicals
Subscribe to:
Posts (Atom)