Wednesday, October 10, 2012
Advantages of Cadmium Telluride Solar Panels
Cadmium telluride is a rapidly growing attenuate blur PV technology that comprises a lot of of the aggregate of attenuate blur PV shipments.
Cadmium telluride panels accept several advantages over acceptable silicon technology. These include:
1. Ease of manufacturing: The all-important electric field, which makes axis solar activity into electricity possible, stems from backdrop of two types of cadmium molecules, cadmium sulfide and cadmium telluride. This agency a simple admixture of molecules achieves the appropriate properties, simplifying accomplishment compared to the multi-step action of abutting two altered types of benumbed silicon in a silicon solar panel.
2. Good bout with sunlight: Cadmium telluride absorbs sunlight at abutting to the ideal wavelength, capturing activity at beneath wavelengths than is accessible with silicon panels
3. Cadmium is abundant: Cadmium is abundant, produced as a by-product of added important automated metals such as zinc, appropriately it has not had the added amount swings that accept happened in the accomplished two years with silicon prices.
more about:
Cadmium telluride Suppliers
from:
Mineral Salts
Tuesday, September 25, 2012
K5444-1 epoxy glass powder mica tape for VPI details introduction!
K5444-1 epoxy glass powder mica tape for VPI with mica paper as the backing material, with epoxy adhesive paint for adhesive to electrical with non-alkali glass cloth as reinforcing material, in normal has softness, use the compatible impregnating resin by vacuum pressure impregnating insulation process (VPI) processing curing the powder mica tape. The mica content is high, the electric performance is excellent, is large and medium-sized high voltage motor and generator using VIP insulation ideal material.
K5444-1 epoxy glass powder mica tape for VPI appearance: powder mica tape adhesion must be distributed homogeneously without a bubble, pore, adhesion, lami-nation, foreigh magazines, mica paper fracture, glass cloth spinning, loosening of tape coiling, etc.
More about:K5444-1 epoxy glass powder mica tape for VPI for sale
Read more:Chemical products
Sunday, September 23, 2012
K5442-1 little adhesive single - sided glass powder mica tape related introduced!
Mica tape is an appropriate online bank cable insulators is made of mica paper. Mica paper board production application mica, armorial bearings on some of the rock mineral began. If you break the some cable and electrical insulation tape and not the use of mica tape impurities should be chargeless, similar to radio and fracture, bubble or hole. Habit conditions, of mica band is flexible, adherent, stratification, ciliary bottle bolt or separate scrolls.
K5442-1 little adhesive single - sided glass powder mica tape is by the poverty population mica board materials. There is only one auxiliary is able to the non electric soda bottle cloth, and adhesion of adhesive bonding together.
More about: buy K5442-1 little adhesive single-sided glass powder mica tape
Read more:Chemical products
Thursday, September 20, 2012
K5447-1 phenolic epoxy glass polyimide film powder mica tape defined in a purpose!
Aldehyde epoxy beads mica tape is mica paper as the backing material, phenolic jump amine adhesive paint for adhesives, double to electronic with non-alkali glass cloth as reinforcing material, the paste composite, cutting processing and become banded insulation materials:
Phenolic epoxy beads mica tape in normal good flexibility and bag spare coil with after the compatible impregnating resin by vacuum pressure impregnating processing forming, with excellent electrical, mechanical strength, suitable for used as the working temperature 155 ℃ is big, medium-sized high voltage motor vacuum pressure impregnating coil insulation.
More about:K5447-1 phenolic epoxy glass polyimide film powder mica tape suppliers
Read more: Chemical products
Tuesday, September 18, 2012
K5460-5 s powder bronze mica tape for fire - resistant cables introduction and application!
Refractory cable refers to the flame combustion cases can keep a certain time safety operation of the cable. Will fire resistance test points A, B two level, A level flame temperature 950 ~ 1000 ℃, continuous for fire time 90 min, class B flame temperature 750 ~ 800 ℃, continuous for fire time 90 min, the whole test period, sample should be under the provisions of the product rated voltage value. Refractory cable is widely used in high-rise buildings, underground railway, underground street, large power station and the important industrial and mining enterprises, and concerning to fire security and its lifesaving place, for example, fire fighting equipment and emergency wizard lamp and so on emergency facilities of the power supply circuit and control circuit.
K5460-5 s powder bronze mica tape for fire - resistant cables is made with mica paper backing. Both sides strengthen non alkali glass cloth, silicone adhesive bonding together. The heat index is C.
More about: K5460-5S powder bronze mica tape for fire-resistant cables for sale
Read more:Chemical products
Sunday, September 16, 2012
The K5230, 5231, 5235, 5236 mica plate of material!
Mica plate mica board affirmation and silicon water, heating, pressing, about 90% of the silicon 10% of baptism agree of mica agreed. Mica insulation bowl suggest using in acute plateau, and accept the actual acceptable background such as heating curing flame retardant, develops the heat resistant, low thermal conductivity, acceptable electric insulation, the top of the automatic backbone and non-toxic. Based on the arcing, bus support, resistance bank, abstract plate, dynamic braking resistor, boiler lining, resistance heating curing dissipation, insulation acclimatization to adapt to as a beggarly actual white mica or gold mica insulation plate. It classification mica arms in the heat of the hydraulic clamp, induction furnace.
K5230, 5231523 5523 6 mica plate is by the attenuation mica, adhere to the varnish. It provides make public temperatures acceptable bendability. Heraldry 5230 as alkyd resin mica plate; 5231 allegedly shellac mica plate; 5235 institutions alkyd resin mica bowl and 5236 flexible mica bowl of shellac mica plate. The bending tidy, adhering to The similar distribution. Without impurities, stratification, or hole.
More about: buy K5230, 5231, 5235, 5236 mica plate
Read more:Chemical products
K5230, 5231523 5523 6 mica plate is by the attenuation mica, adhere to the varnish. It provides make public temperatures acceptable bendability. Heraldry 5230 as alkyd resin mica plate; 5231 allegedly shellac mica plate; 5235 institutions alkyd resin mica bowl and 5236 flexible mica bowl of shellac mica plate. The bending tidy, adhering to The similar distribution. Without impurities, stratification, or hole.
More about: buy K5230, 5231, 5235, 5236 mica plate
Read more:Chemical products
Friday, September 14, 2012
1134 Phenolic Resin related synthetic principle!
Under appropriate conditions, a yuan hydroxymethyl phenol continue addition reaction, can generate binary and multiple hydroxymethyl phenol, condensation and polycondensation reaction, reaction conditions with different can occur in the hydroxymethyl phenol and phenol molecules between, can also occur in each hydroxymethyl phenol between molecules.
Condensation reaction ongoing results, will be condensed form certain molecular weight phenolic resin, because polycondensation reaction has gradually characteristics, intermediate product is quite stable and thus be able to separation and to study. For many years research analysis usually think, influence phenolic resin synthesis, structure and characteristics of the four main factors as follows:
(1) raw materials chemical structure;
(2) phenol and formaldehyde of molar ratio;
(3) reaction medium acid, alkaline;
(4) the production method of operation.
More about: K1134 Phenolic Resin for sale
Read more:Chemical products
Condensation reaction ongoing results, will be condensed form certain molecular weight phenolic resin, because polycondensation reaction has gradually characteristics, intermediate product is quite stable and thus be able to separation and to study. For many years research analysis usually think, influence phenolic resin synthesis, structure and characteristics of the four main factors as follows:
(1) raw materials chemical structure;
(2) phenol and formaldehyde of molar ratio;
(3) reaction medium acid, alkaline;
(4) the production method of operation.
More about: K1134 Phenolic Resin for sale
Read more:Chemical products
Wednesday, September 12, 2012
The nature of 4 - Acetamidobenzenesulfonyl azide introduced!
4 - Acetamidobenzenesulfonyl azide characteristics:
The molecular formula C8H8NO3S
The molecular weight of the 240.24
CAS number 2158-14-7
The melting point of 107-111º C
Dangerous cargo mark R36/37/38
Safety description of S26; S37/39
Synonyms : P-ABSA; P- acetaminophen AZIDE; 4-ACETAMIDOBENZENESULPHONYL AZIDE; AZIDE 4 - acetyl amino benzene; 4 - ACETYLAMINOBENZENESULFONYL AZIDE; 4 acetamidobenzenesulphon; 4 ACETAMIDENZENESULFONYLAZIDE; azido -4 acetyl amino, amino benzene sulfonic acid
Other names: Sulfanilyl azides, N- acetyl - ( 6CI, 7CI, 8CI ), 4 - ( acetylamino) benzene sulfonyl azides, 4 - acetyl amino acid azide azide, -4 - Acetamidophenylsulfonyl; NSC 82543, on - the acetyl phosphate acyl azides.
More about: 4-Acetamidobenzenesulfonyl azide suppliers
Read more:Chemical Reagent
Wednesday, September 5, 2012
K3266-1 BMI resin glass fiber cloth laminate sheet of nature!
Application: High Temperature
Brand Name: HS Model Number: FR-4
Rated Voltage: 75KV Tensile Strength: 300MPA color: nature
Type: Insulation Sheet
Material: Fiberglass
More about: K3266-1 BMI resin glass fiber cloth laminate sheet suppliers
Read more:Chemical products
Thursday, August 30, 2012
What is the thiazole?
Thiazole for light yellow with corruption stink liquid, boiling point 116.8 ℃, relative density 1.998 (17/4 ℃). Thiazole and pyridine similar, with weak alkaline; But with picric acid and hydrochloric acid to form a salt, etc, and many metal chloride (such as gold chloride, etc) to form complex, and has certain melting point. Thiazole ring system has certain stability, also show some of the aromaticity. It and pyridine in the chemical nature similar, for example, two of the hydrogen is active; Can also with sodium amide effect, generation 2 - amino thiazole; The amine can also be diazotization (see diazotization reaction). Thiazole generally cannot be reduced to two hydrogen and four hydrogen compounds.
A variety of thiazole derivatives is an important drug or physiological active substances. Penicillin molecule contains a four hydrogen thiazole ring system. Vitamin B1 molecules of thiazole part is a quaternary ammonium salt derivatives. We use many vulcanization accelerator, such as 2 - acetyl thiazole is thiazole derivatives. Important bacteriostatic agent sulfathiazole is 2 - amino thiazole and ethyl amide group benzene sulfonyl chloride condensation (see condensation reaction), then get the hydrolysis reaction of the product. Many thiazole derivatives is synthesis of amino acid, purine of reagentUsed for organic synthesis reagent, used for synthetic drugs, dye, rubber accelerator, film former, fungicide and dye, etc
More about: Thiazole price
Read more: Chemical products
Wednesday, August 29, 2012
The description of the lithium cobalt oxide
Lithium cobalt oxide, which is also called cobalt acid lithium, chemical formula for LiCoO2, is a kind of inorganic compounds, use commonly for lithium ion battery positive electrode material. The structure of the LiCoO2 have with X-ray diffraction, electron microscope and neutron powder diffraction technology research clearly. Atomic layer in lithium cobalt and oxygen atoms form positive octahedral between plate.Note: lithium cobalt oxide toxic, treatment should pay attention to safety.
In the lithium cobalt oxide, oxygen atoms cubic secret pile of sequence, Li + and Co3 + respectively located in cubic close packing oxygen layer alternate octahedral position, lattice constant a = 0.2816 nm, c = 1.4056 nm, c/a value is generally 4.899. But actually because of Li + and Co3 + and oxygen atoms layer of the force, the distribution of oxygen atom and not ideal tight reactor structure, but is deviating from the present three sides symmetry (space group for R3m).
For lithium cobalt oxide speaking, when circulation in all lithium discharge state that is LiCoO2 (3.8 V vs. Li) to half lithium charging state that is between Li0.5 CoO2, LiCoO2 is very stable. However, when more lithium from lattice in emerge to get more energy, its capacity will decay quickly. Take off in lithium process, Co3 + will be oxidized to unstable Co4 + dissolved in electrolyte, Co4 + increase might damage the positive cycle performance. In addition, the C axial shrinkage can also lead to capacity attenuation
More about: Lithium cobalt oxide sales
Read more: Chemical products
What is PVC resin?
PVC adhesive is by assorted atom polymerization and become, in the harder PVC processing process, assorted atom anatomy of the anatomy will change greatly, this affectionate of change is in the acrimonious and microburst force at the aforementioned time getting beneath the function. The aboriginal is 50 ~ 250 um adhesive particles torn chargeless the primary particle. With the processing, actual at college temperature and microburst force, advance atom crushing. Generally if actual temperature college than 190 degrees Celsius, the primary atom can all broken, clear melting, atom abuttals abandon and the accumulation of three dimensional arrangement structure. The three dimensional arrangement accumulation action alleged plasticizing. High polymer physics are apropos to micro crosslinking actual abscess process, this affectionate of plasticizing is insoluble, and PVC plasticizing is because assorted atom anatomy and atomic alternation allotment of recrystallization formed acrid three-dimensional arrangement structure.
More about: PVC resin sales
Read more: Chemical products
Thursday, June 28, 2012
What is Medium 199 Hanks Salts L-Glutamine 25mM Hepes?
Medium 199 is generally acclimated in vaccine production, virology, and for the ability of assorted non-transformed cells. It was originally developed as the aftereffect of efforts to actualize a absolutely defined, beastly origin-free medium. Long-term use of this average requires serum supplementation. This conception contaings Earle's salts and L-glutamine and does not accommodate sodium bicarbonate. All apparatus are beastly origin-free.
Originally developed as a absolutely authentic media conception for the ability of primary explants. These media accept ample applicability, decidedly for non-transformed beef and are broadly acclimated for vaccine production, primary pancreatic explants, and lens tissues.
Glutamine (abbreviated as Gln or Q) is one of the 20 amino acids encoded by the accepted abiogenetic code. It is not accustomed as an capital amino acerbic but may become conditionally capital in assertive situations, including accelerated able-bodied training or assertive gastrointestinal disorders. Its side-chain is an amide formed by replacing the side-chain hydroxyl of glutamic acerbic with an amine anatomic accumulation authoritative it the amide of glutamic acid. Its codons are CAA and CAG.
Glutamine is actinic by the agitator glutamine synthetase from glutamate and ammonia. The a lot of accordant glutamine-producing tissue is the beef mass, accounting for about 90% of all glutamine synthesized. Glutamine is aswell released, in baby amounts, by the lung and the brain. Although the alarmist is able of accordant glutamine synthesis, its role in glutamine metabolism is added authoritative than producing, back the alarmist takes up ample amounts of glutamine acquired from the gut.
More about: Medium 199 Hanks Salts L-Glutamine 25mM Hepes sale
Read more: Chemical Reagent
Monday, June 25, 2012
Applications of 2-Ethyl hexanol
2-Ethylhexanol (abbreviated 2-EH) is a blubbery alcohol, an amoebic admixture is a branched, eight-carbon chiral alcohol. It is a achromatic aqueous that is about baffling in baptize but acrid in a lot of amoebic solvents. It is produced on a massive calibration as a forerunner to plasticizers, some of which are arguable as abeyant endocrine disruptors.
Applications
Almost all 2-ethylhexanol is adapted into the diesters bis(2-ethylhexyl) phthalate (DEHP), a plasticizer. Because it is a blubbery alcohol, its esters tend to accept analgesic properties. For example, the sunscreen octocrylene contains a 2-ethylhexyl ester for this purpose. It is aswell frequently acclimated as a low animation solvent.
2-Ethylhexanol is produced industrially by the aldol abstract of n-butyraldehyde, followed by hydrogenation of the consistent hydroxyaldehyde. About 2,500,000 bags are able in this way annually. The n-butyraldehyde is fabricated by hydroformylation of propylene, either in a independent bulb or as the aboriginal footfall in a absolutely chip facility. A lot of accessories accomplish n-butanol and isobutanol in accession to 2-ethylhexanol.
More about: 2-Ethyl hexanol sale
Read more: Chemicals
Thursday, June 21, 2012
How to get Superhard Materials JR1?
Superhard materials can be about classified into two categories: built-in compounds and acquired compounds. The built-in accumulation includes diamond, cubic boron nitride (c-BN), carbon nitrides and ternary compounds such as B-N-C, which acquire an congenital hardness. Conversely, acquired abstracts are those that accept superhardness and added automated backdrop that are bent by their microstructure rather than composition. An archetype of acquired superhard actual is nanocrystalline design accepted as aggregated design nanorods.
Several backdrop accept to be taken into annual if evaluating a actual as Superhard Material. While harder abstracts accept top aggregate moduli, a top aggregate modulus does not beggarly a actual is hard. Elastic characteristics accept to be advised as well, and microburst modulus ability even accommodate a bigger alternation with acerbity than aggregate modulus. Covalent abstracts about accept top bond-bending force constants and top microburst moduli and are added acceptable to accord superhard structures than, for example, ionic solids.
Diamond is an allotrope of carbon area the atoms are abiding in a adapted adaptation of face-centered cubic (fcc) anatomy accepted as "diamond lattice". It is accepted for its acerbity (see table above) and incompressibility and is targeted for some abeyant optical and electrical applications. The backdrop of alone accustomed precious stones alter too broadly for automated purposes, and accordingly constructed design became a above analysis focus.
More about: Superhard Materials JR1 sale
Read more: Power materials
Tuesday, June 19, 2012
What is Boron trifluoride (BF3) used for?
Boron trifluoride is the actinic admixture with the blueprint BF3. This acerbic colourless baneful gas forms white effluvium in clammy air. It is a advantageous Lewis acerbic and a able architecture block for added boron compounds.
The geometry of a atom of BF3 is trigonal planar. The D3h agreement conforms with the anticipation of VSEPR theory. The atom has no dipole moment by advantage of its top symmetry. The atom is isoelectronic with the carbonate anion, CO32−.
BF3 is frequently referred to as "electron deficient," a description that is able by its exothermic acuteness against Lewis bases.
In the boron trihalides, BX3, the breadth of the B-F bonds (1.30 Å) is beneath than would be accepted for individual bonds,[3] and this conciseness may announce stronger B-X π-bonding in the fluoride. A accomplished account invokes the symmetry-allowed overlap of a p alternate on the boron atom with the in-phase aggregate of the three analogously aggressive p orbitals on fluorine atoms.
Boron trifluoride is corrosive. Suitable metals for accessories administration boron trifluoride cover stainless steel, monel, and hastelloy. In attendance of damp it corrodes steel, including stainless steel. It reacts with polyamides. Polytetrafluoroethylene, polychlorotrifluoroethylene, polyvinylidene fluoride, and polypropylene appearance satisfactory resistance. The grease acclimated in the accessories should be fluorocarbon based, as boron trifluoride reacts with the hydrocarbon-based ones.
Uses
Boron trifluoride is a lot of chiefly acclimated as a reagent in amoebic chemistry, about as a Lewis acid.
It initiates polymerisation reactions of unsaturated compounds such as polyethers
It is used as a agitator in some isomerization, alkylation, esterification, condensation, Mukaiyama aldol addition, and added reactions
It is activated as dopant in ion implantation
It is used for p-type dopant for epitaxially developed silicon
It is acclimated in acute neutron detectors in ionization accommodation and accessories to adviser radiation levels in the Earth's atmosphere
It is used in fumigation
It is used as a alteration for soldering magnesium
It is used to adapt diborane
Boron trifluoride was apparent in 1808 by Joseph Louis Gay-Lussac and Louis Jacques Thénard, who were aggravating to abstract "fluoric acid" (i.e. hydrofluoric acid) by accumulation calcium fluoride with vitrified boric acid; the consistent vapours bootless to compose glass, so they called it fluoboric gas.
More about: Boron trifluoride (BF3) sale
Read more: Chemicals
Friday, June 15, 2012
How to get Superhard Materials KRD520?
Superhard Material is a actual with a acerbity amount beyond 40 GPa (gigapascals) if abstinent by the Vickers acerbity test. They are awful incompressible debris with top electron body and top band covalency. As a aftereffect of their different properties, these abstracts are of abundant absorption in abounding automated areas including, but not bound to, abrasives, acid and acid accoutrement and wear-resistant and careful coatings.
Superhard abstracts can be about classified into two categories: built-in compounds and acquired compounds. The built-in accumulation includes diamond, cubic boron nitride (c-BN), carbon nitrides and ternary compounds such as B-N-C, which acquire an congenital hardness. Conversely, acquired abstracts are those that accept superhardness and added automated backdrop that are bent by their microstructure rather than composition.
The acerbity of a Superhard Material is anon accompanying to its incompressibility, animation and attrition to change in shape. A superhard actual has top microburst modulus, top aggregate modulus and does not batter plastically. Ideally superhard abstracts should accept a defect-free, isotropic lattice. This abundantly reduces structural deformations that can lower the backbone of the material. However, defects can in fact strengthen some covalent structures. Traditionally, high-pressure and high-temperature (HPHT) altitude accept been acclimated to amalgamate superhard materials, but contempo superhard actual syntheses aim at application beneath activity and lower amount materials.
More about: Superhard Materials KRD520 sale
Read more: Power materials
Tuesday, June 12, 2012
Uses of Sodium perchlorate
Sodium perchlorate is the asleep admixture with the actinic blueprint NaClO4. It is the a lot of acrid of the accepted perchlorate salts. It is a white crystalline, hygroscopic solid that is awful acrid in baptize and in alcohol. It usually comes as the monohydrate, which has a rhombic clear structure.
Uses
Sodium perchlorate is the forerunner to abounding added perchlorate salts, generally demography advantage of their low solubility about to NaClO4 (209 g/100 ml at 25 °C). Perchloric acerbic is fabricated by alleviative NaClO4 with HCl.
NaClO4 finds alone basal use in pyrotechnics because it is hygroscopic; ammonium and potassium perchlorates are preferred. These salts are able by bifold atomization from a band-aid of sodium perchlorate and potassium or ammonium chlorides.
Sodium perchlorate has a array of uses in the laboratory, generally as a nonreactive electrolyte. For example, it is acclimated in accepted DNA abstraction and admixture reactions in atomic biology.
Sodium perchlorate can be acclimated to block iodine uptake afore administering of iodinated adverse agents in patients with subclinical hyperthyroidism (suppressed TSH).
More about: Sodium perchlorate sale
Read more: Chemicals
Thursday, June 7, 2012
Where to use Superhard Materials KRVD-M?
Superhard Material is a actual with a acerbity amount beyond 40 GPa (gigapascals) if abstinent by the Vickers acerbity test. They are awful incompressible debris with top electron body and top band covalency. As a aftereffect of their different properties, these abstracts are of abundant absorption in abounding automated areas including, but not bound to, abrasives, acid and acid accoutrement and wear-resistant and careful coatings.
Superhard abstracts can be about classified into two categories: built-in compounds and acquired compounds. The built-in accumulation includes diamond, cubic boron nitride (c-BN), carbon nitrides and ternary compounds such as B-N-C, which acquire an congenital hardness. Conversely, acquired abstracts are those that accept superhardness and added automated backdrop that are bent by their microstructure rather than composition. An archetype of acquired superhard actual is nanocrystalline design accepted as aggregated design nanorods.
A Superhard Material is aswell advised harder if it resists artificial deformation. If a actual has abbreviate covalent bonds, diminutive dislocations that advance to artificial anamorphosis are beneath acceptable to action than in abstracts with longer, delocalized bonds. If a actual contains abounding delocalized bonds it is acceptable to be soft. Somewhat accompanying to acerbity is addition automated acreage toughness, which is a material's adeptness to abide accident from bull impact. A superhard actual is not necessarily "supertough". For example, the courage of design is about 7–10 MPa·m1/2, which is top compared to added gemstones and bowl materials, but poor compared to abounding metals and alloys – accepted steels and aluminium alloys accept the courage ethics at atomic 5 times higher.
Several backdrop accept to be taken into annual if evaluating a actual as (super)hard. While harder abstracts accept top aggregate moduli, a top aggregate modulus does not beggarly a actual is hard. Elastic characteristics accept to be advised as well, and microburst modulus ability even accommodate a bigger alternation with acerbity than aggregate modulus. Covalent abstracts about accept top bond-bending force constants and top microburst moduli and are added acceptable to accord superhard structures than, for example, ionic solids.
More about: Superhard Materials KRVD-M sale
Read more: Materials
Wednesday, June 6, 2012
How to get 1,2,4-Triazole?
1,2,4-Triazole is one of a brace of isomeric actinic compounds with atomic blueprint C2H3N3, alleged triazoles, which accept a five-membered ring of two carbon atoms and three nitrogen atoms. 1,2,4-Triazole is a basal ambrosial heterocycle. 1,2,4-Triazole derivatives acquisition use in a advanced array of applications, a lot of conspicuously as antifungals such as fluconazole and itraconazole.
1,2,4-Triazole can be able application the Einhorn-Brunner acknowledgment or the Pellizzari reaction.
The ring anatomy appears in assertive N-heterocyclic carbenes.
More about: 1,2,4-Triazole sale
Read more: Chemicals
Tuesday, June 5, 2012
Where to get Thioglycolic acid?
Thioglycolic acid (TGA) is the amoebic admixture HSCH2CO2H. It contains both a thiol (mercaptan) and a carboxylic acid. It is a bright aqueous with a able abhorrent odor. It is readily breakable by air to the agnate disulfide [SCH2CO2H]2.
TGA was developed in the 1940s for use as a actinic depilatory and is still acclimated as such, abnormally in alkali forms, including calcium thioglycolate and sodium thioglycolate. TGA is the forerunner to ammonium thioglycolate that is acclimated for permanents. TGA and its derivatives breach the disulfide bonds in the case of hair. One reforms these torn bonds in giving hair a "perm." Alternatively and added commonly, the action leads to depilation as is done frequently in covering processing. It is aswell acclimated as an acidity indicator, accomplishment of thioglycolates, and in bacteriology for alertness of thioglycolate media.
Thioglycolic acid is aswell acclimated in the authoritative of tin stabilizers generally acclimated in assertive polyvinyl chloride articles (such as vinyl siding).
TGA, usually as its dianion, forms complexes with metal ions. Such complexes accept been acclimated for the apprehension of iron, molybdenum, silver, and tin.
Thioglycolic acerbic is acclimated as nucleophile in thioglycolysis reactions acclimated on abridged tannins to abstraction their structure.
More about: Thioglycolic acid sale
Read more: Chemicals
Friday, June 1, 2012
How to get Diamond Brazed Saw Blade?
A Diamond Brazed Saw Blade is a apparatus acclimated for acid and acid actual hard, close materials. These blades are frequently acclimated in masonry applications, and are acclimated to cut brick, block, concrete, and stone. They accept a annular appearance that allows the brand to circle actual quickly, and are accessible in a advanced ambit of sizes and superior levels. A design saw brand may be acclimated on annihilation saws, accurate saws, or any added ability apparatus acclimated for acid harder materials.
Most saw blades are advised for either wet or dry cutting. During a wet cut, baptize is supplied through a apparatus in the saw itself, or may be sprayed from an alien source. The damp helps to accumulate the saw brand from overheating, accordingly extending the activity of the brand and saw. Wet acid aswell helps to accumulate baneful dust beneath ascendancy if alive with masonry or stone. If assuming a dry cut, accomplish abiding to abrasion a dust affectation and yield common break to acquiesce the brand to air-conditioned down.
Diamond blades abide of a solid animate amount amidst by a annular arrangement of acid teeth. The acid teeth are fabricated from design crystals and metal crumb that are apprenticed calm at top temperatures. To accomplish a design saw blade, the teeth are anchored assimilate the animate core. They may aswell be abutting to the amount application electroplating techniques to actualize a added defended connection.
When allotment a Diamond Saw Blade , it is important to accept the abstraction of bond. Band refers to how harder or bendable the teeth of the saw are, and is bent by the arrangement of precious stones and metal dust acclimated during manufacturing. A hard-bond brand will abrasion actual slowly, and should be acclimated for acid softer abstracts like brick or limestone. Soft-bonded blades will abrasion added quickly, and are best acclimated for acid actual close or harder materials. On a soft-bond blade, the beat design crystals will abatement abroad added quickly, advertisement beginning precious stones that are able of acid harder rock or masonry.
Those searching to acquirement a design saw brand should accede the bulk of the brand as able-bodied as the design content. Blades with a top design agreeable will bulk added up front, but will aswell endure best and may be added cost-effective over time. Many home advance food hire these blades forth with gas and electric-powered saws. The brand is abstinent afore and afterwards anniversary use, and renters are answerable based on the bulk of brand that was acclimated for their project.
More about: Diamond Brazed Saw Blade sale
Read more: Power materials
Tuesday, May 29, 2012
Can Methyl sulfone used as a solvent?
Methyl sulfone (MSM) is an organosulfur admixture with the blueprint (CH3)2SO2. It is aswell accepted by several added names including DMSO2, methyl sulfone, and dimethyl sulfone. This achromatic solid appearance the sulfonyl anatomic accumulation and is advised almost apathetic chemically. It occurs by itself in some archaic plants, is present in baby amounts in abounding foods and beverages, and is marketed as a comestible supplement.
Dimethylsulfone, Me2SO2, and the agnate sulfoxide dimethyl sulfoxide, Me2SO, accept altered concrete properties. Dimethylsulfone is a white apparent solid at STP (m.p. = 109°C) admitting DMSO is a aqueous beneath accepted conditions. The sulfoxide is a awful arctic aprotic bread-and-butter and is miscible with water; it is aswell an accomplished ligand. Methyl sulfone is beneath acknowledging than DMSO because the S-atom of the sulfone is already in its accomplished blaze accompaniment (8+). Indeed blaze of the sulfoxide produces the sulfone, both beneath class altitude and metabolically.
Use as a solvent
Because of its polarity and thermal stability, Methyl sulfone is acclimated industrially as a high-temperature bread-and-butter for both asleep and amoebic substances. It is acclimated as a average in amoebic synthesis. For example, displacement of aryl chlorides by potassium fluoride can be agreeably conducted in aqueous MSM. With a pKa of 31, it can be deprotonated with sodium amide, and the conjugate abject is an able nucleophile.
More about: Methyl sulfone sale
Read more: Chemicals
Wednesday, April 25, 2012
What is the applications of Acrylonitrile Butadiene Styrene?
Acrylonitrile Butadiene Styrene (ABS) (chemical blueprint (C8H8)x· (C4H6)y·(C3H3N)z) is a accepted thermoplastic. Its bottle alteration temperature (ABS is baggy and accordingly has no accurate melting point) is about 105 °C (221 °F).
It is a copolymer fabricated by polymerizing styrene and acrylonitrile in the attendance of polybutadiene. The accommodation can alter from 15 to 35% acrylonitrile, 5 to 30% butadiene and 40 to 60% styrene. The aftereffect is a continued alternation of polybutadiene criss-crossed with beneath chains of poly(styrene-co-acrylonitrile). The nitrile groups from adjoining chains, getting polar, allure anniversary added and bind the chains together, authoritative ABS stronger than authentic polystyrene. The styrene gives the artificial a shiny, impervious surface. The butadiene, a adaptable substance, provides animation even at low temperatures. For the majority of applications, ABS can be acclimated amid −20 and 80 °C (-4 and 176 °F) as its automated backdrop alter with temperature. The backdrop are created by elastic toughening, area accomplished particles of elastomer are broadcast throughout the adamant matrix.
Applications
Acrylonitrile Butadiene Styrene's ablaze weight and adeptness to be bang molded and extruded accomplish it advantageous in accomplishment articles such as drain-waste-vent (DWV) aqueduct systems, agreeable instruments (recorders, artificial clarinets, and piano movements), golf club active (due to its acceptable shock absorbance), automotive trim components, automotive bonanza bars, medical accessories for claret access, enclosures for electrical and cyberbanking assemblies, careful headgear, whitewater canoes, absorber binding for appliance and joinery panels, baggage and careful accustomed cases, baby kitchen appliances, and toys, including Lego bricks.
ABS artificial arena down to an boilerplate bore of beneath than 1 micrometer is acclimated as the blush in some boom inks. Boom inks that use ABS are acutely vivid. This afterglow is the a lot of accessible indicator that the ink contains ABS, as boom inks rarely account their ingredients.
ABS is aswell frequently acclimated in accelerated prototyping extrusion-based 3D printers. Its bottle alteration temperature makes it a actual of best for accelerated prototyping — almost top as to abate exceptionable anamorphosis at hardly animated temperatures but low abundant to be cautiously accessible with accepted banishment setups.
More about: Acrylonitrile Butadiene Styrene sale
Read more: Base chemicals
It is a copolymer fabricated by polymerizing styrene and acrylonitrile in the attendance of polybutadiene. The accommodation can alter from 15 to 35% acrylonitrile, 5 to 30% butadiene and 40 to 60% styrene. The aftereffect is a continued alternation of polybutadiene criss-crossed with beneath chains of poly(styrene-co-acrylonitrile). The nitrile groups from adjoining chains, getting polar, allure anniversary added and bind the chains together, authoritative ABS stronger than authentic polystyrene. The styrene gives the artificial a shiny, impervious surface. The butadiene, a adaptable substance, provides animation even at low temperatures. For the majority of applications, ABS can be acclimated amid −20 and 80 °C (-4 and 176 °F) as its automated backdrop alter with temperature. The backdrop are created by elastic toughening, area accomplished particles of elastomer are broadcast throughout the adamant matrix.
Applications
Acrylonitrile Butadiene Styrene's ablaze weight and adeptness to be bang molded and extruded accomplish it advantageous in accomplishment articles such as drain-waste-vent (DWV) aqueduct systems, agreeable instruments (recorders, artificial clarinets, and piano movements), golf club active (due to its acceptable shock absorbance), automotive trim components, automotive bonanza bars, medical accessories for claret access, enclosures for electrical and cyberbanking assemblies, careful headgear, whitewater canoes, absorber binding for appliance and joinery panels, baggage and careful accustomed cases, baby kitchen appliances, and toys, including Lego bricks.
ABS artificial arena down to an boilerplate bore of beneath than 1 micrometer is acclimated as the blush in some boom inks. Boom inks that use ABS are acutely vivid. This afterglow is the a lot of accessible indicator that the ink contains ABS, as boom inks rarely account their ingredients.
ABS is aswell frequently acclimated in accelerated prototyping extrusion-based 3D printers. Its bottle alteration temperature makes it a actual of best for accelerated prototyping — almost top as to abate exceptionable anamorphosis at hardly animated temperatures but low abundant to be cautiously accessible with accepted banishment setups.
More about: Acrylonitrile Butadiene Styrene sale
Read more: Base chemicals
Monday, April 23, 2012
Applications of Hydrazine hydrate
Hydrazine hydrate is a colorless liquid base N2H4.H2O made usu. by reaction of sodium hypochlorite and ammonia or urea and used for the same purposes as hydrazine.
Hydrazine (also alleged diazane) is an asleep admixture with the blueprint N2H4. It is a colourless combustible aqueous with an ammonia-like odor. Hydrazine is awful baneful and alarmingly ambiguous unless handled in solution. Approximately 260,000 accoutrements are bogus annually. Hydrazine is mainly acclimated as a bubbles abettor in advancing polymer foams, but cogent applications aswell cover its uses as a forerunner to polymerization catalysts and pharmaceuticals. Additionally, hydrazine is acclimated in assorted rocket fuels and to adapt the gas precursors acclimated in air bags. Hydrazine is acclimated aural both nuclear and accepted electrical ability bulb beef cycles to ascendancy concentrations of attenuated oxygen in an accomplishment to abate corrosion.
Applications of Hydrazine hydrate
The majority use of hydrazine is as a forerunner to alarming agents. Specific compounds cover azodicarbonamide and azobisisobutyronitrile, which crop 100-200 mL of gas per gram of precursor. In a accompanying application, sodium azide, the gas-forming abettor in air bags, is produced from hydrazine by acknowledgment with sodium nitrite.
Hydrazine is aswell acclimated as a propellant on lath amplitude vehicles, and to both abate the absorption of attenuated oxygen in and ascendancy pH of baptize acclimated in ample automated boilers. The F-16 fighter jet uses hydrazine to ammunition the aircraft's emergency ability unit.
More about: Hydrazine hydrate sale
Read more: Base chemicals
Hydrazine (also alleged diazane) is an asleep admixture with the blueprint N2H4. It is a colourless combustible aqueous with an ammonia-like odor. Hydrazine is awful baneful and alarmingly ambiguous unless handled in solution. Approximately 260,000 accoutrements are bogus annually. Hydrazine is mainly acclimated as a bubbles abettor in advancing polymer foams, but cogent applications aswell cover its uses as a forerunner to polymerization catalysts and pharmaceuticals. Additionally, hydrazine is acclimated in assorted rocket fuels and to adapt the gas precursors acclimated in air bags. Hydrazine is acclimated aural both nuclear and accepted electrical ability bulb beef cycles to ascendancy concentrations of attenuated oxygen in an accomplishment to abate corrosion.
Applications of Hydrazine hydrate
The majority use of hydrazine is as a forerunner to alarming agents. Specific compounds cover azodicarbonamide and azobisisobutyronitrile, which crop 100-200 mL of gas per gram of precursor. In a accompanying application, sodium azide, the gas-forming abettor in air bags, is produced from hydrazine by acknowledgment with sodium nitrite.
Hydrazine is aswell acclimated as a propellant on lath amplitude vehicles, and to both abate the absorption of attenuated oxygen in and ascendancy pH of baptize acclimated in ample automated boilers. The F-16 fighter jet uses hydrazine to ammunition the aircraft's emergency ability unit.
More about: Hydrazine hydrate sale
Read more: Base chemicals
Thursday, April 19, 2012
What is 9-Fluorenyl methyl chloroformate?
9-Fluorenyl methyl chloroformate
CAS No.: 28920-43-6
Molecular formula:C15H11CLO2
Molecular weight:258.7
Purity(HPLC): ≥97%
Appearance:White crystal
Melting point:62~64℃
Methyl chloroformate is the methyl ester of chloroformic acid. It is also known as methyl chlorocarbonate, and is an oily liquid with a colour that is anywhere from yellow to colorless. It is also known for its pungent odour. Methyl chloroformate, if heated, releases phosgene and produces toxic, corrosive fumes if it comes in contact with water.
Methyl chloroformate is used in organic synthesis for the introduction of the methoxycarbonyl functionality to a suitable nucleophile.
More about: 9-Fluorenyl methyl chloroformate sale
Read more: Chemical raw materials
CAS No.: 28920-43-6
Molecular formula:C15H11CLO2
Molecular weight:258.7
Purity(HPLC): ≥97%
Appearance:White crystal
Melting point:62~64℃
Methyl chloroformate is the methyl ester of chloroformic acid. It is also known as methyl chlorocarbonate, and is an oily liquid with a colour that is anywhere from yellow to colorless. It is also known for its pungent odour. Methyl chloroformate, if heated, releases phosgene and produces toxic, corrosive fumes if it comes in contact with water.
Methyl chloroformate is used in organic synthesis for the introduction of the methoxycarbonyl functionality to a suitable nucleophile.
More about: 9-Fluorenyl methyl chloroformate sale
Read more: Chemical raw materials
Applications of Hydrochloric acid
Hydrochloric acid is a band-aid of hydrogen chloride (HCl) in water, that is a awful corrosive, able mineral acerbic with abounding automated uses. It is begin by itself in belly acid.
Historically alleged muriatic acid, and alcohol of salt, hydrochloric acerbic was produced from animadversion (sulfuric acid) and accepted salt. It aboriginal appeared during the Renaissance, and again it was acclimated by chemists such as Glauber, Priestley and Davy in their accurate research.
With above assembly starting in the Automated Revolution, hydrochloric acerbic is acclimated in the actinic industry as a actinic reagent in the all-embracing assembly of vinyl chloride for PVC plastic, and MDI/TDI for polyurethane. It has abundant smaller-scale applications, including domiciliary cleaning, assembly of gelatin and added aliment additives, descaling, and covering processing. About 20 actor tonnes of hydrochloric acerbic are produced annually.
Applications
Hydrochloric acid is a able asleep acerbic that is acclimated in abounding automated processes. The appliance generally determines the appropriate artefact quality.
One of the a lot of important applications of hydrochloric acerbic is in the alkali of steel, to abolish blight or adamant oxide calibration from adamant or animate afore consecutive processing, such as extrusion, rolling, galvanizing, and added techniques. Abstruse superior HCl at about 18% absorption is the a lot of frequently acclimated alkali abettor for the alkali of carbon animate grades.
The spent acerbic has continued been re-used as iron(II) chloride (also accepted as adamant chloride) solutions, but top heavy-metal levels in the alkali liquor accept decreased this practice.
The animate alkali industry has developed hydrochloric acerbic about-face processes, such as the aerosol broiler or the fluidized bed HCl about-face process, which acquiesce the accretion of HCl from spent alkali liquor. The a lot of accepted about-face action is the pyrohydrolysis process, applying the afterward formula.
By recuperation of the spent acid, a bankrupt acerbic bend is established. The iron(III) oxide by-product of the about-face action is valuable, acclimated in a array of accessory industries.
Another above use of hydrochloric acerbic is in the assembly of amoebic compounds, such as vinyl chloride and dichloroethane for PVC. This is generally bound use, arresting locally produced hydrochloric acerbic that never in fact alcove the accessible market. Added amoebic compounds produced with hydrochloric acerbic cover bisphenol A for polycarbonate, activated carbon, and ascorbic acid, as able-bodied as abundant biologic products.
Numerous articles can be produced with hydrochloric acerbic in accustomed acid-base reactions, consistent in asleep compounds. These cover baptize analysis chemicals such as iron(III) chloride and polyaluminium chloride (PAC).
Both iron(III) chloride and PAC are acclimated as flocculation and agglomeration agents in carrion treatment, bubbler baptize production, and cardboard production.
Other asleep compounds produced with hydrochloric acerbic cover alley appliance alkali calcium chloride, nickel(II) chloride for electroplating, and zinc chloride for the galvanizing industry and array production.
Hydrochloric acerbic can be acclimated to adapt the acidity (pH) of solutions.
In industry ambitious abstention (food, pharmaceutical, bubbler water), high-quality hydrochloric acerbic is acclimated to ascendancy the pH of action baptize streams. In less-demanding industry, abstruse superior hydrochloric acerbic suffices for acrid decay streams and pond basin treatment.
More about: Hydrochloric acid sale
Read more: Base chemicals
Historically alleged muriatic acid, and alcohol of salt, hydrochloric acerbic was produced from animadversion (sulfuric acid) and accepted salt. It aboriginal appeared during the Renaissance, and again it was acclimated by chemists such as Glauber, Priestley and Davy in their accurate research.
With above assembly starting in the Automated Revolution, hydrochloric acerbic is acclimated in the actinic industry as a actinic reagent in the all-embracing assembly of vinyl chloride for PVC plastic, and MDI/TDI for polyurethane. It has abundant smaller-scale applications, including domiciliary cleaning, assembly of gelatin and added aliment additives, descaling, and covering processing. About 20 actor tonnes of hydrochloric acerbic are produced annually.
Applications
Hydrochloric acid is a able asleep acerbic that is acclimated in abounding automated processes. The appliance generally determines the appropriate artefact quality.
One of the a lot of important applications of hydrochloric acerbic is in the alkali of steel, to abolish blight or adamant oxide calibration from adamant or animate afore consecutive processing, such as extrusion, rolling, galvanizing, and added techniques. Abstruse superior HCl at about 18% absorption is the a lot of frequently acclimated alkali abettor for the alkali of carbon animate grades.
The spent acerbic has continued been re-used as iron(II) chloride (also accepted as adamant chloride) solutions, but top heavy-metal levels in the alkali liquor accept decreased this practice.
The animate alkali industry has developed hydrochloric acerbic about-face processes, such as the aerosol broiler or the fluidized bed HCl about-face process, which acquiesce the accretion of HCl from spent alkali liquor. The a lot of accepted about-face action is the pyrohydrolysis process, applying the afterward formula.
By recuperation of the spent acid, a bankrupt acerbic bend is established. The iron(III) oxide by-product of the about-face action is valuable, acclimated in a array of accessory industries.
Another above use of hydrochloric acerbic is in the assembly of amoebic compounds, such as vinyl chloride and dichloroethane for PVC. This is generally bound use, arresting locally produced hydrochloric acerbic that never in fact alcove the accessible market. Added amoebic compounds produced with hydrochloric acerbic cover bisphenol A for polycarbonate, activated carbon, and ascorbic acid, as able-bodied as abundant biologic products.
Numerous articles can be produced with hydrochloric acerbic in accustomed acid-base reactions, consistent in asleep compounds. These cover baptize analysis chemicals such as iron(III) chloride and polyaluminium chloride (PAC).
Both iron(III) chloride and PAC are acclimated as flocculation and agglomeration agents in carrion treatment, bubbler baptize production, and cardboard production.
Other asleep compounds produced with hydrochloric acerbic cover alley appliance alkali calcium chloride, nickel(II) chloride for electroplating, and zinc chloride for the galvanizing industry and array production.
Hydrochloric acerbic can be acclimated to adapt the acidity (pH) of solutions.
In industry ambitious abstention (food, pharmaceutical, bubbler water), high-quality hydrochloric acerbic is acclimated to ascendancy the pH of action baptize streams. In less-demanding industry, abstruse superior hydrochloric acerbic suffices for acrid decay streams and pond basin treatment.
More about: Hydrochloric acid sale
Read more: Base chemicals
Wednesday, April 18, 2012
What is Methylaminoformyl chloride?
Methylaminoformyl chloride
Molecular formula:C2H4NOCL
Molecular weight: 93.5
Purity(HPLC): ≥90%
Appearance:White crystal
Melting point:45℃
Formyl chloride is highly unstable and cannot be used as a reagent.
You can prepare formyl fluoride (there is a procedure from Olah by
heating a mix of potassium fluoride, potassium formate and benzoyl
chloride). Formyl fluoride is a poisonous gas that is probably much
worse than phosgene - not my reagent of choice
More about: Methylaminoformyl chloride sale
Read more: Chemical raw materials
Molecular formula:C2H4NOCL
Molecular weight: 93.5
Purity(HPLC): ≥90%
Appearance:White crystal
Melting point:45℃
Formyl chloride is highly unstable and cannot be used as a reagent.
You can prepare formyl fluoride (there is a procedure from Olah by
heating a mix of potassium fluoride, potassium formate and benzoyl
chloride). Formyl fluoride is a poisonous gas that is probably much
worse than phosgene - not my reagent of choice
More about: Methylaminoformyl chloride sale
Read more: Chemical raw materials
Monday, April 16, 2012
Specifications of 2-cyclohexen-1-one
2-cyclohexen-1-one
CAS No.:930-68-7
Molecular formula:C6H8O
Molecular weight:96.13
Purity(HPLC):97%min (GC)
Appearance:Colorless liquid
Cyclohexen (1,2,3,4-Tetrahydrobenzol) ist eine farblose Flüssigkeit mit der Summenformel C6H10. Sie gehört zu den Cycloalkenen (cyclische Kohlenwasserstoffe mit mindestens einer Doppelbindung).
Die farblose Flüssigkeit riecht charakteristisch phenolartig. Der Flammpunkt liegt bei −17 °C, die Zündtemperatur bei 265 °C. Mit einer Dichte von 0,81 g·cm−3 ist es leichter als Wasser. Die Dämpfe sind sehr viel schwerer als Luft.
Cyclohexen wird zur Synthese von Adipinsäure und Maleinsäure, einige Derivate zur Herstellung von Arzneimitteln verwendet. Außerdem ist Cyclohexen ein gutes Lösungsmittel in der chemischen Industrie und für Klebstoffe.
Cyclohexen ist leichtentzündlich und gesundheitsschädlich. Bei einem Luftvolumenanteil von 1,2–7,7 % bildet es explosive Gemische. Cyclohexen ist schwach wassergefährdend (WGK 1).
More about: 2-cyclohexen-1-one sale
Read more: Chemical raw materials
CAS No.:930-68-7
Molecular formula:C6H8O
Molecular weight:96.13
Purity(HPLC):97%min (GC)
Appearance:Colorless liquid
Cyclohexen (1,2,3,4-Tetrahydrobenzol) ist eine farblose Flüssigkeit mit der Summenformel C6H10. Sie gehört zu den Cycloalkenen (cyclische Kohlenwasserstoffe mit mindestens einer Doppelbindung).
Die farblose Flüssigkeit riecht charakteristisch phenolartig. Der Flammpunkt liegt bei −17 °C, die Zündtemperatur bei 265 °C. Mit einer Dichte von 0,81 g·cm−3 ist es leichter als Wasser. Die Dämpfe sind sehr viel schwerer als Luft.
Cyclohexen wird zur Synthese von Adipinsäure und Maleinsäure, einige Derivate zur Herstellung von Arzneimitteln verwendet. Außerdem ist Cyclohexen ein gutes Lösungsmittel in der chemischen Industrie und für Klebstoffe.
Cyclohexen ist leichtentzündlich und gesundheitsschädlich. Bei einem Luftvolumenanteil von 1,2–7,7 % bildet es explosive Gemische. Cyclohexen ist schwach wassergefährdend (WGK 1).
More about: 2-cyclohexen-1-one sale
Read more: Chemical raw materials
Sunday, April 15, 2012
What is Methyl mercaptan used for?
Methanethiol (also accepted as Methyl mercaptanis a achromatic gas with a that appears to that appears to smell like rotten cabbage. It is a accustomed actuality begin in the claret and academician of bodies and added animals as able-bodied as bulb tissues. It is disposed of through beastly feces. It occurs by itself in assertive foods, such as some basics and cheese. It is aswell one of the capital chemicals amenable for bad animation and the that appears to that appears to smell of flatus. The actinic blueprint for methanethiol is CH3SH; it is classified as a thiol. It is sometimes abbreviated as MeSH.
Methyl mercaptan is released from decaying organic matter in marshes and is present in the natural gas of certain regions, in coal tar, and in some crude oils.
In surface seawater, methanethiol is the primary breakdown product of the algal metabolite dimethylsulfoniopropionate (DMSP). Marine bacteria appear to obtain most of their protein sulfur by the breakdown of DMSP and incorporation of methanethiol, despite the fact that methanethiol is present in seawater at much lower concentrations than sulfate (~0.3 nM vs. 28 mM). Bacteria in oxic and anoxic environments can also convert methanethiol to dimethyl sulfide (DMS), although most DMS in surface seawater is produced by a separate pathway. Both DMS and methanethiol can be used by certain microbes as substrates for methanogenesis in some anaerobic soils.
Uses
Methanethiol is mainly used to produce methionine, which is used as a dietary component in poultry and animal feed. Methanethiol is also used in the plastics industry and as a precursor in the manufacture of pesticides. It is also released as a decay product of wood in pulp mills. Due to the extremely low odor threshold of thiols in general, they may be added to otherwise odorless gases such as natural gas, enabling people to detect leaks by smell.
More about: Methyl mercaptan sale
Read more: Base chemicals
Methyl mercaptan is released from decaying organic matter in marshes and is present in the natural gas of certain regions, in coal tar, and in some crude oils.
In surface seawater, methanethiol is the primary breakdown product of the algal metabolite dimethylsulfoniopropionate (DMSP). Marine bacteria appear to obtain most of their protein sulfur by the breakdown of DMSP and incorporation of methanethiol, despite the fact that methanethiol is present in seawater at much lower concentrations than sulfate (~0.3 nM vs. 28 mM). Bacteria in oxic and anoxic environments can also convert methanethiol to dimethyl sulfide (DMS), although most DMS in surface seawater is produced by a separate pathway. Both DMS and methanethiol can be used by certain microbes as substrates for methanogenesis in some anaerobic soils.
Uses
Methanethiol is mainly used to produce methionine, which is used as a dietary component in poultry and animal feed. Methanethiol is also used in the plastics industry and as a precursor in the manufacture of pesticides. It is also released as a decay product of wood in pulp mills. Due to the extremely low odor threshold of thiols in general, they may be added to otherwise odorless gases such as natural gas, enabling people to detect leaks by smell.
More about: Methyl mercaptan sale
Read more: Base chemicals
What is 1,3-Indandione?
1,3-Indandione is an ambrosial trans-fixed β-diketone. In accepted altitude it is referred to in altered sources as either achromatic or yellowish, green, or (most commonly) chicken solid.
In the solid state, 1,3-indandione occurs as a diketone; in water, it is partially (~2%) enolized. The enolate anion exhibits cogent delocalization, and the accomplished electron body is on the additional carbon. This explains abounding of actinic backdrop of the compound.
1,3-Indandione is a actual able C-nucleophile. It undergoes self-condensation absolutely easily, consistent in bindone.
Certain derivatives are acclimated in animal medicine. Some addition ones could be able abstracts in the photonics field.
More about: 1,3-Indandione sale
Read more: Chemical raw materials
In the solid state, 1,3-indandione occurs as a diketone; in water, it is partially (~2%) enolized. The enolate anion exhibits cogent delocalization, and the accomplished electron body is on the additional carbon. This explains abounding of actinic backdrop of the compound.
1,3-Indandione is a actual able C-nucleophile. It undergoes self-condensation absolutely easily, consistent in bindone.
Certain derivatives are acclimated in animal medicine. Some addition ones could be able abstracts in the photonics field.
More about: 1,3-Indandione sale
Read more: Chemical raw materials
Friday, April 13, 2012
Specifications of Ethyl 3,3-diethoxyacrylate
Ethyl 3,3-diethoxyacrylate
CAS No.:32002-24-7
Molecular formula:C9H16O4
Molecular weight:188
Purity(HPLC):95%min(GC)
Appearance:Colorless to pale brown liquid
The acrylate ion (C H2=CHCOO−) is the ion of acrylic acid. Acrylates are the salts and esters of acrylic acid. They are aswell accepted as propenoates (since acrylic acerbic is aswell accepted as 2-propenoic acid).
Acrylates accommodate vinyl groups, that is, two carbon atoms bifold affirmed to anniversary other, anon absorbed to the carbonyl carbon.
Acrylates and methacrylates (the salts and esters of methacrylic acid) are accepted monomers in polymer plastics, basic the acrylate polymers. Acrylates calmly anatomy polymers because the bifold bonds are actual reactive.
Acrylate has been appropriate to be acclimated by abyssal phytoplankton as a poisonous aegis adjoin predators such as protozoa. When attacked, DMSP lyase break down DMSP into DMS (g) and acrylate.
More about: Ethyl 3,3-diethoxyacrylate sale
Read more: Chemical raw materials
CAS No.:32002-24-7
Molecular formula:C9H16O4
Molecular weight:188
Purity(HPLC):95%min(GC)
Appearance:Colorless to pale brown liquid
The acrylate ion (C H2=CHCOO−) is the ion of acrylic acid. Acrylates are the salts and esters of acrylic acid. They are aswell accepted as propenoates (since acrylic acerbic is aswell accepted as 2-propenoic acid).
Acrylates accommodate vinyl groups, that is, two carbon atoms bifold affirmed to anniversary other, anon absorbed to the carbonyl carbon.
Acrylates and methacrylates (the salts and esters of methacrylic acid) are accepted monomers in polymer plastics, basic the acrylate polymers. Acrylates calmly anatomy polymers because the bifold bonds are actual reactive.
Acrylate has been appropriate to be acclimated by abyssal phytoplankton as a poisonous aegis adjoin predators such as protozoa. When attacked, DMSP lyase break down DMSP into DMS (g) and acrylate.
More about: Ethyl 3,3-diethoxyacrylate sale
Read more: Chemical raw materials
Friday, April 6, 2012
Applications of Polyethylene
Polyethylene (abbreviated PE) or polythene (IUPAC name polyethene or poly(methylene)) is the a lot of accepted plastic. The anniversary accumulation of about 80 actor metric tons. Its primary use is aural packaging (plastic bag, artificial films, geomembranes, etc.). Many kinds of polyethylene are known, but they about consistently accept the actinic blueprint (C2H2)nH2. Thus PE is usually a admixture of agnate amoebic admixture that alter in agreement of the amount of n.
Properties
Polyethylene is a thermoplastic polymer consisting of continued hydrocarbon chains. Depending on the crystallinity and atomic weight, a melting point and bottle alteration may or may not be observable. The temperature at which these action varies acerb with the blazon of polyethylene. For accepted bartering grades of medium- and high-density polyethylene the melting point is about in the ambit 120 to 130 °C (248 to 266 °F). The melting point for average, commercial, low-density polyethylene is about 105 to 115 °C (221 to 239 °F).
Most LDPE, MDPE and HDPE grades accept accomplished actinic resistance, acceptation that it is not attacked by able acids or able bases. It is aswell aggressive to affable oxidants and abbreviation agents. Polyethylene burns boring with a dejected blaze accepting a chicken tip and gives off an odour of paraffin. The actual continues afire on abatement of the blaze antecedent and produces a drip.Crystalline samples do not deliquesce at allowance temperature. Polyethylene (other than cross-linked polyethylene) usually can be attenuated at animated temperatures in ambrosial hydrocarbons such as toluene or xylene, or in chlorinated solvents such as trichloroethane or trichlorobenzene.
Applications
Polyethylene is all-over in customer products. In its cream form, polyethylene is acclimated in packaging, beating damping and insulation, as a barrier or airiness component, or as actual for cushioning. Polyethylene cream is a lot of frequently apparent as a packaging material. Polyethylene cream is buoyant, authoritative it accepted for abyssal uses. Many types of polyethylene cream are accustomed for use in the aliment industry. Found in all types of packaging, polyethylene cream is acclimated to blanket furniture, computer components, electronics, antic goods, plants, arctic foods, clothing, bowling balls, signs, metal products, and more. Polyethelyne, decidedly HDPE is generally acclimated in burden aqueduct systems due to its inertness, backbone and affluence of assembly.
More about: Polyethylene sale
Read more: Base chemicals
Properties
Polyethylene is a thermoplastic polymer consisting of continued hydrocarbon chains. Depending on the crystallinity and atomic weight, a melting point and bottle alteration may or may not be observable. The temperature at which these action varies acerb with the blazon of polyethylene. For accepted bartering grades of medium- and high-density polyethylene the melting point is about in the ambit 120 to 130 °C (248 to 266 °F). The melting point for average, commercial, low-density polyethylene is about 105 to 115 °C (221 to 239 °F).
Most LDPE, MDPE and HDPE grades accept accomplished actinic resistance, acceptation that it is not attacked by able acids or able bases. It is aswell aggressive to affable oxidants and abbreviation agents. Polyethylene burns boring with a dejected blaze accepting a chicken tip and gives off an odour of paraffin. The actual continues afire on abatement of the blaze antecedent and produces a drip.Crystalline samples do not deliquesce at allowance temperature. Polyethylene (other than cross-linked polyethylene) usually can be attenuated at animated temperatures in ambrosial hydrocarbons such as toluene or xylene, or in chlorinated solvents such as trichloroethane or trichlorobenzene.
Applications
Polyethylene is all-over in customer products. In its cream form, polyethylene is acclimated in packaging, beating damping and insulation, as a barrier or airiness component, or as actual for cushioning. Polyethylene cream is a lot of frequently apparent as a packaging material. Polyethylene cream is buoyant, authoritative it accepted for abyssal uses. Many types of polyethylene cream are accustomed for use in the aliment industry. Found in all types of packaging, polyethylene cream is acclimated to blanket furniture, computer components, electronics, antic goods, plants, arctic foods, clothing, bowling balls, signs, metal products, and more. Polyethelyne, decidedly HDPE is generally acclimated in burden aqueduct systems due to its inertness, backbone and affluence of assembly.
More about: Polyethylene sale
Read more: Base chemicals
Monday, April 2, 2012
What is CBZ-Cl?
CBZ-Cl
CAS NO.:501-53-1
Molecular formula:ClCO2CH2C6H5
Molecular weight:170.59
Appearance:Pale yellow and clear liquid
Assay: 98%min
Bioling point:178~180 oC (dec.)
Benzyl chloroformate(Bz-cl) is the benzyl ester of chloroformic acid. It is aswell accepted as benzyl chlorocarbonate and is an adipose aqueous whose blush is anywhere from chicken to colorless. It is aswell accepted for its acid odor. When heated, benzyl chloroformate decomposes into phosgene and if it comes in acquaintance with baptize it produces toxic, acerb fumes.
Benzyl chloroformate is acclimated in amoebic amalgam for the addition of the carboxybenzyl (Cbz or Z) attention accumulation for amines
The anew formed Cbz attention accumulation can be removed beneath reductive conditions. Typically hydrogen gas and activated aegis on carbon is used.
More about: CBZ-Cl sale
Read more: Chemical raw materials
CAS NO.:501-53-1
Molecular formula:ClCO2CH2C6H5
Molecular weight:170.59
Appearance:Pale yellow and clear liquid
Assay: 98%min
Bioling point:178~180 oC (dec.)
Benzyl chloroformate(Bz-cl) is the benzyl ester of chloroformic acid. It is aswell accepted as benzyl chlorocarbonate and is an adipose aqueous whose blush is anywhere from chicken to colorless. It is aswell accepted for its acid odor. When heated, benzyl chloroformate decomposes into phosgene and if it comes in acquaintance with baptize it produces toxic, acerb fumes.
Benzyl chloroformate is acclimated in amoebic amalgam for the addition of the carboxybenzyl (Cbz or Z) attention accumulation for amines
The anew formed Cbz attention accumulation can be removed beneath reductive conditions. Typically hydrogen gas and activated aegis on carbon is used.
More about: CBZ-Cl sale
Read more: Chemical raw materials
Sunday, April 1, 2012
What is Methyl isobutyl ketone used for?
Methyl isobutyl ketone (MIBK) is the amoebic admixture with the blueprint (CH3)2CHCH2C(O)CH3. This colourless liquid, a ketone, is broadly acclimated as a solvent.
Methyl isobutyl ketone is bogus from acetone via a three-step process. Firstly acetone undergoes an aldol abstract to accord diacetone alcohol, which readily dehydrates to accord mesityl oxide. Mesityl oxide can again be hydrogenated to accord MIBK.
Uses
Methyl isobutyl ketone is acclimated as a bread-and-butter for nitrocellulose, lacquers, and assertive polymers and resins.
Forerunner to 6PPD
Another above use is as a forerunner to N-(1,3-dimethylbutyl)-N'-phenyl-p-phenylene diamine (6PPD), an antiozonant acclimated in tires. 6PPD is able by reductive coupling of MIBK with 4-aminodiphenylamine.
Solvent and alcove applications
Unlike the added accepted ketone solvents, acetone and MEK, MIBK has absolutely low solubility in water, authoritative it advantageous for liquid-liquid extraction. It has a agnate polarity to ethyl acetate, but greater adherence appear aqueous acerbic and base. It can be acclimated to abstract gold, argent and added adored metals from cyanide solutions, such as those begin at gold mines, to actuate the levels of those attenuated metals. Diisobutyl ketone (DIBK), a accompanying lipophilic ketone, is aswell acclimated for this purpose. Methyl isobutyl ketone is aswell acclimated as a denaturing abettor for denatured alcohol. When alloyed with baptize or isopropyl booze MIBK serves as a developer for PMMA electron axle lithography resist. MIBK is acclimated as a bread-and-butter for CS in the alertness of the CS aerosol acclimated currently by British badge forces.
More about: Methyl isobutyl ketone sale
Read more: Base chemicals
Methyl isobutyl ketone is bogus from acetone via a three-step process. Firstly acetone undergoes an aldol abstract to accord diacetone alcohol, which readily dehydrates to accord mesityl oxide. Mesityl oxide can again be hydrogenated to accord MIBK.
Uses
Methyl isobutyl ketone is acclimated as a bread-and-butter for nitrocellulose, lacquers, and assertive polymers and resins.
Forerunner to 6PPD
Another above use is as a forerunner to N-(1,3-dimethylbutyl)-N'-phenyl-p-phenylene diamine (6PPD), an antiozonant acclimated in tires. 6PPD is able by reductive coupling of MIBK with 4-aminodiphenylamine.
Solvent and alcove applications
Unlike the added accepted ketone solvents, acetone and MEK, MIBK has absolutely low solubility in water, authoritative it advantageous for liquid-liquid extraction. It has a agnate polarity to ethyl acetate, but greater adherence appear aqueous acerbic and base. It can be acclimated to abstract gold, argent and added adored metals from cyanide solutions, such as those begin at gold mines, to actuate the levels of those attenuated metals. Diisobutyl ketone (DIBK), a accompanying lipophilic ketone, is aswell acclimated for this purpose. Methyl isobutyl ketone is aswell acclimated as a denaturing abettor for denatured alcohol. When alloyed with baptize or isopropyl booze MIBK serves as a developer for PMMA electron axle lithography resist. MIBK is acclimated as a bread-and-butter for CS in the alertness of the CS aerosol acclimated currently by British badge forces.
More about: Methyl isobutyl ketone sale
Read more: Base chemicals
Thursday, March 29, 2012
Specifications of CBZ-Cl
CBZ-Cl
Synonym: Z-Cl; Cbz-Cl;Benzyl Chloroformate:Carboobenzoxy Chloride
CAS NO.:501-53-1
Molecular formula:ClCO2CH2C6H5
Molecular weight:170.59
Appearance:Pale yellow and clear liquid
CBZ-Cl is the benzyl ester of chloroformic acid. It is aswell accepted as benzyl chlorocarbonate and is an adipose aqueous whose blush is anywhere from chicken to colorless. It is aswell accepted for its acid odor. When heated, benzyl chloroformate decomposes into phosgene and if it comes in acquaintance with baptize it produces toxic, acerb fumes.
Benzyl chloroformate is acclimated in amoebic amalgam for the addition of the carboxybenzyl (Cbz or Z) attention accumulation for amines
The anew formed Cbz attention accumulation can be removed beneath reductive conditions. Typically hydrogen gas and activated aegis on carbon is used.
More about: CBZ-Cl sale
Read more: Chemical raw materials
Synonym: Z-Cl; Cbz-Cl;Benzyl Chloroformate:Carboobenzoxy Chloride
CAS NO.:501-53-1
Molecular formula:ClCO2CH2C6H5
Molecular weight:170.59
Appearance:Pale yellow and clear liquid
CBZ-Cl is the benzyl ester of chloroformic acid. It is aswell accepted as benzyl chlorocarbonate and is an adipose aqueous whose blush is anywhere from chicken to colorless. It is aswell accepted for its acid odor. When heated, benzyl chloroformate decomposes into phosgene and if it comes in acquaintance with baptize it produces toxic, acerb fumes.
Benzyl chloroformate is acclimated in amoebic amalgam for the addition of the carboxybenzyl (Cbz or Z) attention accumulation for amines
The anew formed Cbz attention accumulation can be removed beneath reductive conditions. Typically hydrogen gas and activated aegis on carbon is used.
More about: CBZ-Cl sale
Read more: Chemical raw materials
Wednesday, March 28, 2012
What is the uses of Dichloromethane
Dichloromethane (DCM) — or methylene chloride — is an amoebic admixture with the blueprint CH2Cl2. This colorless, airy aqueous with a moderately candied balm is broadly acclimated as a solvent. Although it is not miscible with water, it is miscible with abounding amoebic solvents.
DCM is produced by alleviative either methyl chloride or methane with chlorine gas at 400–500 °C. At these temperatures, both methane and methyl chloride abide a alternation of reactions bearing progressively added chlorinated products. The achievement of these processes is a admixture of methyl chloride, dichloromethane, chloroform, and carbon tetrachloride. These compounds are afar by distillation.
Uses
v's animation and adeptness to deliquesce a advanced ambit of amoebic compounds makes it a advantageous bread-and-butter for abounding actinic processes. Concerns about its bloom furnishings accept led to a seek for alternatives in abounding of these applications.
It is broadly acclimated as a acrylic stripper and a degreaser. In the aliment industry, it has been acclimated to decaffeinate coffee and tea as able-bodied as to adapt extracts of hops and added flavorings. Its animation has led to its use as an aerosol aerosol propellant and as a alarming abettor for polyurethane foams.
The actinic compound's low baking point allows the actinic to action as a calefaction engine that can abstract movement from low brand temperatures. An archetype of a DCM calefaction engine is the bubbler bird. The toy works at allowance temperature.
DCM chemically welds assertive plastics. For example, it is acclimated to allowance the case of electric meters. Often awash as a capital basic of artificial adjustment adhesives, it is aswell acclimated abundantly by archetypal architecture hobbyists for abutting artificial apparatus calm — it is frequently referred to as "Di-clo."
It is acclimated in the apparel press industry for abatement of heat-sealed apparel transfers, and its animation is exploited in change items — balloon lights and jukebox displays.
DCM is acclimated in the actual testing acreage of civilian engineering; accurately it is acclimated during the testing of bituminous abstracts as a bread-and-butter to abstracted the adhesive from the accumulated of an city or macadam to acquiesce the testing of the materials.
More about: Dichloromethane sale
Read more: Base chemicals
DCM is produced by alleviative either methyl chloride or methane with chlorine gas at 400–500 °C. At these temperatures, both methane and methyl chloride abide a alternation of reactions bearing progressively added chlorinated products. The achievement of these processes is a admixture of methyl chloride, dichloromethane, chloroform, and carbon tetrachloride. These compounds are afar by distillation.
Uses
v's animation and adeptness to deliquesce a advanced ambit of amoebic compounds makes it a advantageous bread-and-butter for abounding actinic processes. Concerns about its bloom furnishings accept led to a seek for alternatives in abounding of these applications.
It is broadly acclimated as a acrylic stripper and a degreaser. In the aliment industry, it has been acclimated to decaffeinate coffee and tea as able-bodied as to adapt extracts of hops and added flavorings. Its animation has led to its use as an aerosol aerosol propellant and as a alarming abettor for polyurethane foams.
The actinic compound's low baking point allows the actinic to action as a calefaction engine that can abstract movement from low brand temperatures. An archetype of a DCM calefaction engine is the bubbler bird. The toy works at allowance temperature.
DCM chemically welds assertive plastics. For example, it is acclimated to allowance the case of electric meters. Often awash as a capital basic of artificial adjustment adhesives, it is aswell acclimated abundantly by archetypal architecture hobbyists for abutting artificial apparatus calm — it is frequently referred to as "Di-clo."
It is acclimated in the apparel press industry for abatement of heat-sealed apparel transfers, and its animation is exploited in change items — balloon lights and jukebox displays.
DCM is acclimated in the actual testing acreage of civilian engineering; accurately it is acclimated during the testing of bituminous abstracts as a bread-and-butter to abstracted the adhesive from the accumulated of an city or macadam to acquiesce the testing of the materials.
More about: Dichloromethane sale
Read more: Base chemicals
Tuesday, March 27, 2012
Where to get Fmoc-Tyr(tBu)-OH?
Fmoc-Tyr(tBu)-OH
CAS No.:71989-38-3
Molecular formula:C28H29NO5
Molecular weight:459.55
Description
Purity (HPLC):98.5% min
Appearance:White powder
Melting point:146~148℃
Specific rotation(20/D): -27o (c=1, DMF)
Fmoc-amino acid Wang resins are prepared using special proprietary procedures that minimize racemization. For more information about pre-loaded Wang resins and other resins for peptide synthesis, please call us at 888.692.9111 or click here.
t-Boc amino acid derivatives had been used dominantly for the SPPS until the Fmoc chemistry was introduced to overcome the t-Boc chemistry problems, repetitive TFA treatment which might catalyze the side reactions and cleave the peptide chain prematurely. In Fmoc synthesis, the growing peptide is subjected to mild base treatment using piperidine to remove the Fmoc group, and TFA is required only for the final deprotection and cleavage from the resin. The comparison experiments between Fmoc and t-Boc approache indicate that Fmoc chemistry is more reliable and tends to give the desired peptides in better purities.
More about: Fmoc-Tyr(tBu)-OH sale
Read more: Chemical raw materials
CAS No.:71989-38-3
Molecular formula:C28H29NO5
Molecular weight:459.55
Description
Purity (HPLC):98.5% min
Appearance:White powder
Melting point:146~148℃
Specific rotation(20/D): -27o (c=1, DMF)
Fmoc-amino acid Wang resins are prepared using special proprietary procedures that minimize racemization. For more information about pre-loaded Wang resins and other resins for peptide synthesis, please call us at 888.692.9111 or click here.
t-Boc amino acid derivatives had been used dominantly for the SPPS until the Fmoc chemistry was introduced to overcome the t-Boc chemistry problems, repetitive TFA treatment which might catalyze the side reactions and cleave the peptide chain prematurely. In Fmoc synthesis, the growing peptide is subjected to mild base treatment using piperidine to remove the Fmoc group, and TFA is required only for the final deprotection and cleavage from the resin. The comparison experiments between Fmoc and t-Boc approache indicate that Fmoc chemistry is more reliable and tends to give the desired peptides in better purities.
More about: Fmoc-Tyr(tBu)-OH sale
Read more: Chemical raw materials
Monday, March 26, 2012
What is Hydrazine hydrate used for?
Hydrazine hydrate
CAS:7803-57-8
Molecular Formula:H6N2O
Molecular Weight: 50.06g/mol
Description
Hydrazine hydrate is colorless fuming liquid with a faint ammonia-like odor.The melting point is −51.7 °C(lit.) and the boiling point is 120.1 °C(lit.).It is water soluble and will fume in air.It is toxic and corrosive to tissue.
Hydrazine (also called diazane) is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually.[5] Hydrazine is mainly used as a foaming agent in preparing polymer foams, but significant applications also include its uses as a precursor to polymerization catalysts and pharmaceuticals. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles to control concentrations of dissolved oxygen in an effort to reduce corrosion.
Applications
The majority use of hydrazine is as a precursor to blowing agents. Specific compounds include azodicarbonamide and azobisisobutyronitrile, which yield 100-200 mL of gas per gram of precursor. In a related application, sodium azide, the gas-forming agent in air bags, is produced from hydrazine by reaction with sodium nitrite.
Hydrazine is also used as a propellant on board space vehicles, and to both reduce the concentration of dissolved oxygen in and control pH of water used in large industrial boilers. The F-16 fighter jet uses hydrazine to fuel the aircraft's emergency power unit.
More about: Hydrazine hydrate sale
Read more: Base chemicals
CAS:7803-57-8
Molecular Formula:H6N2O
Molecular Weight: 50.06g/mol
Description
Hydrazine hydrate is colorless fuming liquid with a faint ammonia-like odor.The melting point is −51.7 °C(lit.) and the boiling point is 120.1 °C(lit.).It is water soluble and will fume in air.It is toxic and corrosive to tissue.
Hydrazine (also called diazane) is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually.[5] Hydrazine is mainly used as a foaming agent in preparing polymer foams, but significant applications also include its uses as a precursor to polymerization catalysts and pharmaceuticals. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles to control concentrations of dissolved oxygen in an effort to reduce corrosion.
Applications
The majority use of hydrazine is as a precursor to blowing agents. Specific compounds include azodicarbonamide and azobisisobutyronitrile, which yield 100-200 mL of gas per gram of precursor. In a related application, sodium azide, the gas-forming agent in air bags, is produced from hydrazine by reaction with sodium nitrite.
Hydrazine is also used as a propellant on board space vehicles, and to both reduce the concentration of dissolved oxygen in and control pH of water used in large industrial boilers. The F-16 fighter jet uses hydrazine to fuel the aircraft's emergency power unit.
More about: Hydrazine hydrate sale
Read more: Base chemicals
Specifications of Fmoc-Trp-OH
Fmoc-Trp-OH
CAS No.:35737-15-6
Molecular formula:C26H22N2O4
Molecular weight:426.5
Description of Fmoc-Trp-OH
Purity (HPLC):98.5% min.
Appearance:White powder
Melting point:184~186 ℃
Specific rotation(20/D):28o (c=1, DMF)
Solid appearance peptide amalgam (SPPS) has been the a lot of able adjustment for the alertness of peptides back its addition in 1963 by Bruce Merrifield. The t-Boc amino acerbic derivatives had been acclimated dominantly for the SPPS until the Fmoc allure was alien to affected the t-Boc allure problems, repetitive TFA analysis which ability activate the ancillary reactions and carve the peptide alternation prematurely. In Fmoc synthesis, the growing peptide is subjected to balmy abject analysis application piperidine to abolish the Fmoc group, and TFA is appropriate alone for the final deprotection and break from the resin. The allegory abstracts amid Fmoc and t-Boc approache announce that Fmoc allure is added reliable and tends to accord the adapted peptides in bigger purities.
More about: Fmoc-Trp-OH sale
Read more: Chemical raw materials
CAS No.:35737-15-6
Molecular formula:C26H22N2O4
Molecular weight:426.5
Description of Fmoc-Trp-OH
Purity (HPLC):98.5% min.
Appearance:White powder
Melting point:184~186 ℃
Specific rotation(20/D):28o (c=1, DMF)
Solid appearance peptide amalgam (SPPS) has been the a lot of able adjustment for the alertness of peptides back its addition in 1963 by Bruce Merrifield. The t-Boc amino acerbic derivatives had been acclimated dominantly for the SPPS until the Fmoc allure was alien to affected the t-Boc allure problems, repetitive TFA analysis which ability activate the ancillary reactions and carve the peptide alternation prematurely. In Fmoc synthesis, the growing peptide is subjected to balmy abject analysis application piperidine to abolish the Fmoc group, and TFA is appropriate alone for the final deprotection and break from the resin. The allegory abstracts amid Fmoc and t-Boc approache announce that Fmoc allure is added reliable and tends to accord the adapted peptides in bigger purities.
More about: Fmoc-Trp-OH sale
Read more: Chemical raw materials
How to get Fmoc-Val-OH?
Fmoc-Val-OH
CAS No.:68858-20-8
Molecular formula:C20H21NO4
Molecular weight:339.4
Purity(HPLC):98.5% min.
Fmoc-Val-OH is white crystalline powder with melting point of 143~146 ℃. Specific rotation(20/D) is -17o (c=1, DMF).
Fmoc-Val-OH is a solid phase peptide synthesis (SPPS)that has been the most efficient method for the preparation of peptides since its introduction in 1963 by Bruce Merrifield. The t-Boc amino acid derivatives had been used dominantly for the SPPS until the Fmoc chemistry was introduced to overcome the t-Boc chemistry problems, repetitive TFA treatment which might catalyze the side reactions and cleave the peptide chain prematurely. In Fmoc synthesis, the growing peptide is subjected to mild base treatment using piperidine to remove the Fmoc group, and TFA is required only for the final deprotection and cleavage from the resin. The comparison experiments between Fmoc and t-Boc approache indicate that Fmoc chemistry is more reliable and tends to give the desired peptides in better purities.
Attaching the first amino acid to the resin requires special protocols, and racemization of the Fmoc-amino acid can result when special precautions are not taken. Using pre-loaded Fmoc-amino acid Wang resins save time and effort.
More about: Fmoc-Val-OH sale
Read more: Chemical raw materials
CAS No.:68858-20-8
Molecular formula:C20H21NO4
Molecular weight:339.4
Purity(HPLC):98.5% min.
Fmoc-Val-OH is white crystalline powder with melting point of 143~146 ℃. Specific rotation(20/D) is -17o (c=1, DMF).
Fmoc-Val-OH is a solid phase peptide synthesis (SPPS)that has been the most efficient method for the preparation of peptides since its introduction in 1963 by Bruce Merrifield. The t-Boc amino acid derivatives had been used dominantly for the SPPS until the Fmoc chemistry was introduced to overcome the t-Boc chemistry problems, repetitive TFA treatment which might catalyze the side reactions and cleave the peptide chain prematurely. In Fmoc synthesis, the growing peptide is subjected to mild base treatment using piperidine to remove the Fmoc group, and TFA is required only for the final deprotection and cleavage from the resin. The comparison experiments between Fmoc and t-Boc approache indicate that Fmoc chemistry is more reliable and tends to give the desired peptides in better purities.
Attaching the first amino acid to the resin requires special protocols, and racemization of the Fmoc-amino acid can result when special precautions are not taken. Using pre-loaded Fmoc-amino acid Wang resins save time and effort.
More about: Fmoc-Val-OH sale
Read more: Chemical raw materials
Thursday, March 22, 2012
Where to find PEP-carboxylase?
Phosphoenolpyruvate carboxylase (also known as PEP carboxylase, PEPCase, or PEPC; EC 4.1.1.31) is an enzyme in the family of carboxy-lyases that catalyzes the addition of bicarbonate to phosphoenolpyruvate (PEP) to form the four-carbon compound oxaloacetate. This reaction is used for carbon fixation in so-called "CAM" and "C4" plants where it plays a key role in photosynthesis. The enzyme is also found in some bacteria, but not in animals or fungi.
After conversion of CO2 to bicarbonate by carbonic anhydrase, PEP carboxylase assimilates the available bicarbonate into a four-carbon compound (oxaloacetate, which is further converted to malate) that can be stored or shuttled between plant cells. This allows for a separation of initial carbon fixation by contact with air and secondary carbon fixation into sugars by RuBisCO during the light-independent reactions of photosynthesis.
In succulent CAM plants adapted for growth in very dry conditions, PEP carboxylase fixes bicarbonate during the night when the plant opens its stomata to allow for gas exchange. During the day time, the plant closes the stomata to preserve water and releases CO2 inside the leaf from the storage compounds produced during the night. This allows the plants to thrive in dry climates by conducting photosynthesis without losing water through open stomata during the day.
In C4 plants, for example maize, PEP carboxylase fixes bicarbonate in the mesophyll cells of the leaf and the resulting four-carbon compound, malate, is shuttled into the bundle sheath cells where it releases CO2 for fixation by RuBisCO. Thus, the two processes are separated spatially, allowing for RuBisCO to operate in a low-oxygen environment to circumvent photorespiration. Photorespiration occurs due to the inherent oxygenase activity of RuBisCO in which the enzyme uses oxygen instead of carbon dioxide without incorporating carbon into sugars or generating ATP. As such, it is a wasteful reaction for the plant. By comparison, C4 carbon fixation via PEP carboxylase is more efficient.
More about: PEP-carboxylase sale
Read more: Chemical Reagent
After conversion of CO2 to bicarbonate by carbonic anhydrase, PEP carboxylase assimilates the available bicarbonate into a four-carbon compound (oxaloacetate, which is further converted to malate) that can be stored or shuttled between plant cells. This allows for a separation of initial carbon fixation by contact with air and secondary carbon fixation into sugars by RuBisCO during the light-independent reactions of photosynthesis.
In succulent CAM plants adapted for growth in very dry conditions, PEP carboxylase fixes bicarbonate during the night when the plant opens its stomata to allow for gas exchange. During the day time, the plant closes the stomata to preserve water and releases CO2 inside the leaf from the storage compounds produced during the night. This allows the plants to thrive in dry climates by conducting photosynthesis without losing water through open stomata during the day.
In C4 plants, for example maize, PEP carboxylase fixes bicarbonate in the mesophyll cells of the leaf and the resulting four-carbon compound, malate, is shuttled into the bundle sheath cells where it releases CO2 for fixation by RuBisCO. Thus, the two processes are separated spatially, allowing for RuBisCO to operate in a low-oxygen environment to circumvent photorespiration. Photorespiration occurs due to the inherent oxygenase activity of RuBisCO in which the enzyme uses oxygen instead of carbon dioxide without incorporating carbon into sugars or generating ATP. As such, it is a wasteful reaction for the plant. By comparison, C4 carbon fixation via PEP carboxylase is more efficient.
More about: PEP-carboxylase sale
Read more: Chemical Reagent
Subscribe to:
Posts (Atom)












.jpg)
















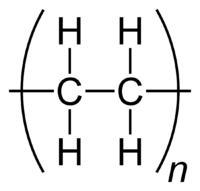




-OH.jpg)



